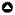The following is the established format for referencing this article:
Jernakoff, M. G., J. L. Knowlton, B. Vásquez-Ávila, C. I. Espinosa and B. A. Tinoco 2023. Effects of land use change on the functional diversity and composition of mixed species avian flocks in the high tropical Andes of southern Ecuador. Journal of Field Ornithology 94(1):3.ABSTRACT
One of the greatest threats to biodiversity is land use change. Habitat alteration can have strong impacts on functional diversity, i.e., the range of biological traits within a suite of organisms. Mixed species avian flocks are integral to maintaining both taxonomic and functional diversity in tropical forests as they provide participants with greater foraging efficiency, reduced predation risks, and increased resiliency of species and ecosystems to environmental change. Our aim for this study was to determine the effects of land use change on the taxonomic and functional structures of mixed species avian flocks in and around Cajas National Park, Azuay Province in the tropical Andes of southern Ecuador by comparing non-native vegetation in agricultural plots and forest regenerating from historical use as pastureland to the largest intact primary forest in the region. We found no changes in species richness and diversity or functional richness of flocks across habitats, but we did find significant differences in species composition. Surprisingly, we found higher functional diversity and uniqueness of flocks in regenerating forest with more diverse and structurally complex vegetation, but native and non-native forests were functionally similar. We did, however, find significant differences in the community weighted means of four of five functional traits in different habitat types. Overall, the taxonomic and functional structures of mixed species flocks in this region seem relatively unaffected by land use change, indicating flocking as a beneficial strategy for species with higher sensitivities to disturbance in areas with anthropogenic activities. It is critical to maintain functional diversity in ecosystems, as a greater variety of functional traits and functional redundancy implies an efficient and more resilient ecosystem in the face of environmental change. Mixed species flocks provide membership benefits that may mitigate impacts of disturbance via land use change and thus can be considered essential units for conserving avian biodiversity.
RESUMEN
Una de las grandes amenazas a la biodiversidad es el cambio en el uso del suelo. La alteración de los hábitats puede tener impactos fuertes sobre la diversidad funcional, i.e., el rango de características biológicas en un conjunto de organismos. Las bandadas mixtas son integrales para mantener la diversidad taxonómica y funcional en bosques tropicales pues proveen a sus participantes una mayor eficiencia de forrajeo, disminución en el riesgo de depredación e incremento en la resiliencia de las especies y los ecosistemas al cambio ambiental. Nuestro objetivo en este estudio fue determinar los efectos del cambio en el uso del suelo sobre la estructura taxonómica y funcional de bandadas mixtas en y alrededor del Parque Nacional Cajas, Provincia de Azuay en los Andes tropicales en el sur de Ecuador, por medio de la comparación de la vegetación no nativa en plots de agricultura y bosque en regeneración con uso histórico de pasturas con el bosque primario intacto mas grande de la región. No encontramos cambios en la riqueza de especies ni en la diversidad o la riqueza funcional de las bandadas entre hábitats, pero encontramos diferencias en la composición de especies. Sorpresivamente, encontramos una mayor diversidad funcional y unicidad en las bandadas en bosque en regeneración con una vegetación mas diversa y estructuralmente compleja, pero los bosques nativos y no nativos fueron funcionalmente similares. Sin embargo, encontramos diferencias significativas en la media ponderada de la comunidad para cuatro de los cinco caracteres funcionales en hábitats diferentes. En general, la estructura taxonómica y funcional de las bandadas mixtas en esta región parecen estar relativamente no afectadas por los cambios en el uso del suelo, indicando que la formación de bandadas es una estrategia beneficial para las especies con alta sensibilidad al disturbio en áreas con actividades antropogénicas. Es critico mantener la diversidad funcional en los ecosistemas, debido a que una mayor variedad de caracteres funcionales y redundancia funcional implica un ecosistema eficiente y mas resiliente de miras al cambio ambiental. Las bandadas mixtas proveen beneficios a sus miembros que pueden mitigar los impactos del disturbio a través del cambio en el uso del suelo y por lo tanto pueden ser consideradas como unidades esenciales para la conservación de la biodiversidad aviar.
INTRODUCTION
Biodiversity is increasingly threatened around the globe as human populations grow and natural environments are altered (Vitousek 1997, Gaston et al. 2003, Di Marco et al. 2019, Díaz et al. 2019). This is problematic as biodiversity underpins the wide range of supporting, regulating, provisioning, and cultural ecosystem services that benefit humanity (Whelan et al. 2008, Clements et al. 2021) and stabilize ecosystem structures and functions necessary for life on Earth (Cardinale et al. 2012). Land use change is one of the primary threats to biodiversity and associated functional traits (Gaston et al. 2003, Pereira et al. 2012, Díaz et al. 2019), defined as the phenotypic characteristics that influence an organism’s response or tolerance to the environment and its ecosystem level processes (Petchey and Gaston 2006, Cadotte et al. 2011). Land use change impacts the presence and evenness of certain functional traits, such as the loss of specialized species (Pocock et al. 2011, Şekercioḡlu 2012, Coetzee and Chown 2016), often leading to the homogenization of ecosystems and the functional diversity contained within (Weideman et al. 2020).
Functional diversity, or the value and variety of species’ traits that influence ecosystem functioning, is increasingly used as a measure of biodiversity and ecosystem stability (Tilman 2001, Petchey and Gaston 2006). Biodiversity and functional diversity are considered to be linked as the variety of functional traits in a habitat might correspond to the number of available niches (Reiss et al. 2009, Cadotte et al. 2011). Ecosystems with high functional diversity contain species that fill more functional roles, and available resources are used more efficiently (Cadotte et al. 2011, Cardinale et al. 2012). These systems can be more stable as highly diverse communities can compensate for the loss of one species by the presence of another with similar roles and can buffer ecosystems in the face of environmental change (Walker et al. 1999, Díaz and Cabido 2001). To understand impacts of land use change on biodiversity, birds have been proposed as useful indicators (Pereira and Cooper 2006, Gardner et al. 2008) as they comprise a wide range of ecological functions across trophic levels (Järvinen and Väisänen 1979, Şekercioḡlu 2006).
Mixed species flocks of birds occur in many ecosystems and contain numerous species from a variety of genera (Morse 1977, Harrison and Whitehouse 2011). Nearly 20% of the world’s bird species participate in mixed flocks, including 38% of tropical species (Zou et al. 2018). Members derive benefits from flocking, such as increased foraging efficiency and reduced predation risks (Morse 1977, Hutto 1994, Goodale and Kotagama 2005, Sridhar et al. 2009), and interspecific competition associated with foraging in a group is often mediated in mixed flocks due to species with diverse functional traits, foraging guilds, and foraging strategies (Dri et al. 2022). Flocking behavior not only increases the fitness of participants but can allow habitat-specialist species to utilize previously inaccessible or degraded areas via interspecific facilitative interactions (Morse 1977, Jullien and Clobert 2000, Mammides et al. 2015). These flocks are typically composed of ‘nuclear’ (flock leaders) and ‘satellite’ (flock followers) species (Morse 1970, Sridhar et al. 2009). Nuclear species initiate formation and maintain cohesion of flocks through their specific morphological (e.g., body size and shape), physiological (e.g., visual and auditory acuity), and behavioral traits (e.g., gregarious and loud; Goodale et al. 2010), and satellite species will often not participate in flocks if a nuclear species is absent (Hutto 1994, Maldonado-Coelho and Marini 2004, Harrison and Whitehouse 2011).
Habitat alteration can impact mixed flock structure, function, and the likelihood of species joining and participating (Lee et al. 2005, Knowlton and Graham 2011, Zhang et al. 2013). Losing these important interspecific interactions can affect the fitness and distribution of flocking species in a habitat and can potentially alter community composition and the diversity of functional traits related to ecosystem functioning and stability (Cadotte et al. 2011, Goodale et al. 2015). Such changes may be reflected in altered niche occupancy and resource use by a mixed flock assemblage or avian community as a whole (Mason et al. 2005, Rocha et al. 2019). Several studies have analyzed the taxonomic and/or functional diversity of mixed species avian flocks, examining the effects of habitat fragmentation and disturbance within fragments (Jones and Robinson 2020), effects of flock size and environmental covariates (Dri et al. 2022), and effects of an elevational gradient (Zhang et al. 2020). Since functional diversity increases resilience to environmental change (Díaz and Cabido 2001, Díaz et al. 2013), it can be inferred that mixed flocks with greater functional diversity will be less susceptible to impacts of habitat degradation and alteration.
The aim of this study was to investigate the impacts of land use change and resulting alterations to native forest on the taxonomic and functional structures of mixed species avian flocks in the tropical Andes of southern Ecuador. We collected data on mixed flocks in three habitat types with different stages and intensities of land usage: protected native forest, forest regenerating after a history of cattle grazing, and non-native forest consisting primarily of eucalyptus trees (Eucalyptus sp.) and, to a lesser extent, pine trees (Pinus radiata) bordering agricultural and pastoral fields. For taxonomic structure, we compared species richness, diversity, and composition of flocks across habitat types. For functional structure, we compared functional richness (the amount of functional space occupied by species in a flock), diversity, and uniqueness (the average rarity of a species’ traits relative to other species in a flock) using traits related to foraging, movement, and habitat use, and we identified differences in the community weighted means (CWM: mean trait value of all the species that are part of a flock weighted by their abundances) for each trait across habitat types. Our hypothesis was that greater land use change and habitat disturbance would lead to a reduction in the functional diversity of mixed species flocks, potentially making them more vulnerable to collapse in the future. Therefore, we predicted that mixed species flocks in native forest would have greater species richness and diversity, functional richness and diversity, and more functionally unique species than in the regenerating and non-native forests. We also predicted that there would be significant differences in species composition of flocks with turnover among habitat types. The exact differences in the direction and scale of CWMs for functional traits are more difficult to predict as different habitat components can filter for different traits (Kraft and Ackerly 2010). Understanding how anthropogenic activities can alter avian flock dynamics is important for evaluating ecosystem functioning and stability as flock assemblages can show negative impacts before they become apparent at the species or community levels (Goodale et al. 2015, Valiente-Banuet et al. 2015, Borah et al. 2018).
METHODS
Study sites
This study was conducted in Cajas National Park (CNP) and surrounding areas, located in Azuay province in the high tropical Andes mountains of southern Ecuador. This national park ranges in elevation from 3,160 to 4,445 m and encompasses 29,477 ha, including numerous valleys carved out by Pleistocene glaciers (Cordero 2002). This region has an annual precipitation ranging from 1,200 to 1,500 mm in a bimodal pattern, with a main rainy season from January to June, a dry season from July to September, and a secondary rainy season from October to December (Celleri et al. 2007). Mean monthly temperature ranges from 5 to 12 °C.
Established in 1996, CNP is listed as an important bird and biodiversity area (IBA) and is essential for Andean avian conservation as it provides crucial habitats for high montane forest and páramo birds (ETAPA 2018, BirdLife International 2021). Much of the land surrounding CNP has been converted for pastoral and agricultural use, increasing the need for its continued protection. Three valleys with different habitat types were selected as study sites according to land use history and degree of native vegetation: Mazán (native forest), Llaviuco (regenerating forest), and Sayausí (non-native forest) valleys (Fig. 1).
The three habitat types contained varying degrees of primary forest and heterogeneity in vegetation resulting from different land use histories (Vásquez-Ávila et al. 2021). The native forest was located in Mazán, a narrow valley encompassing 2,257 ha of intact primary forest and a low proportion of secondary forest including 300 species of vascular plants, 101 of which are woody species (Minga 2000, Cordero 2002). Granted protected status in 1984, the Mazán valley and reserve is used for research purposes, but otherwise all human interference is prohibited (Chacón-Vintimilla 2016). The Mazán site represents the reference site for the study area as it is the largest continuous native forest in this region. The regenerating forest was located in Llaviuco, a wide U-shaped valley with a history of intense land use and deforestation for cattle ranching until its incorporation into CNP in 1996 (Chacón-Vintimilla 2016). Extensive grasslands and a mix of re-establishing native and non-native vegetation, including arboreal species, shrubs, and low vegetation, characterize the Llaviuco valley (Cordero 2002). While now protected from use as pastoral and agricultural land, human recreational activities such as hiking and fishing are still allowed in designated areas. The third habitat type, non-native forest located in Sayausí valley, possesses relatively little native vegetation, having been mostly replaced with agricultural land and livestock pastures (Cordero 2002). The remaining vegetation within the valley contains some native vegetation intermixed with non-native trees, dominated by non-native eucalyptus trees (Eucalyptus sp.) and, to a lesser extent, pine trees (Pinus radiata) in stands and as windbreaks.
Data collection
Mixed species flocks
Four 500 m transects, spaced 250 m apart to avoid repeated counts of the same flocks (Colorado and Rodewald 2015), were selected in each of the three habitat types for flock observations from June to October 2018. Two observers walked the transects together and recorded observational data between 06:00-10:00 and 15:00-17:00. The observers walked for approximately 40 minutes, stopping for three to five minutes every 100 m along the transect. The observers searched for mixed species flocks (e.g., Fig. 2), defined as individuals of two or more species traveling together in search of food (Morse 1970, Hutto 1994, Sridhar et al. 2009), for at least five minutes (Latta and Wunderle 1996). Upon detection of a flock, the participating species and number of individuals were recorded, and the flock was followed for a maximum of 15 min to ensure complete detection of the flock members. Flocks at high elevations in this region are relatively small, and this time period should be sufficient to detect all members (Hutto 1987, Matthysen et al. 2008). Each transect was sampled for four consecutive days (one sample period), and each transect had five sampling periods. The starting time at each transect varied among the multiple visits to the same transect to account for potential variation in bird activity throughout the day. Details of the data collection can be found in Vásquez-Ávila et al. (2021).
Functional traits
Morphometric characteristics of the species participating in mixed species flocks were measured during mist-netting campaigns in and around CNP (Latta et al. 2011, Tinoco et al. 2019) and supplemented with measurements on museum specimens collected in South America from the Harvard Museum of Comparative Zoology in Cambridge, MA, USA (Table S1). Additional traits were gathered from previous literature to fill in several data gaps (Hellmayr 1925, Wilman et al. 2014). Body mass, bill width, and the lengths of the total culmen, primaries, secondaries, tail, and tarsus were measured as these functional traits influence responses to environmental changes (Petchey and Gaston 2006, Kennedy et al. 2019). Body mass, used here to represent body size, influences many aspects of life history and can be a predictor of abundance in a habitat (Peters 1983, Lewis et al. 2008). Bill morphology relates to both diet and foraging strategies (Friedman et al. 2019), and different foraging strategies and habitat usage have known associations with tail (Thomas and Balmford 1995), wing (Sheard et al. 2020), and leg morphologies (Zeffer et al. 2003).
To account for correlations between certain functional traits, four trait indices (bill index, hand-wing index (HWI), tail index, and tarsus index) were used instead to standardize values across species for more meaningful comparisons. Body mass and the four indices represent the functional traits selected for all subsequent analyses (Table 1). These five functional traits, which are strongly affected by habitat type and foraging stratum, reflect differences in foraging strategies, movement, and habitat usage of flocking species.
Data analysis
Taxonomic descriptors of flocks
All data analyses for this study were performed in R version 4.0.5 (R Development Core Team 2021). The taxonomic structure of each flock was characterized by species richness (the number of species), flock size (the number of individuals), and species diversity (Simpson index); for taxonomic diversity indices we used the vegan package in R (Oksanen et al. 2020). We then explored the influence of habitat type on these taxonomic descriptors with generalized (for species richness) and linear (for all the other descriptors) mixed models. Habitat type was used as a fixed factor in the models, and flocks observed on the same transect and during the same sampling period were used as the random components to account for potential pseudoreplication issues. Additionally, flocks detected on different days and within different sampling periods can be counted as independent replicates due to the open-membership nature of Andean mixed flocks (Poulsen et al. 1996, Montaño-Centellas and Jones 2021). In the case of the species richness, model significance was based on a likelihood ratio test by comparing our model with a null model containing only the intercept, using a Chi-square distribution; for the other model, significance was detected with an ANOVA test using the Satterthwaite method (Kuznetsova et al. 2017). Post-hoc pairwise differences among habitat types were estimated by least square means with P values of less than 0.05 to determine significant differences, using the function LS-means in the package lmerTest in R (Kuznetsova et al. 2017).
We also performed a canonical correspondence analysis (CCA) to explore differences in species composition of flocks. CCA is an ordination method that allows exploring the effects of environmental predictors on changes in the composition of species of sites (Ter Braak 1986). In our case, we used habitat type as a predictor variable and tested its effects on species composition by Monte Carlo permutation test. Rare species are poorly represented on CCA, and therefore we removed from this analysis species with less than four occurrences among flocks. To improve the distribution of the abundances of the different species, abundance data was log-transformed.
Functional descriptors of flocks
The functional structure of flocks was calculated from the information provided by five functional traits measured for each species using metrics that consider different aspects of flocks: functional richness, functional diversity, and functional uniqueness. Functional richness measures the minimum convex polygon in the functional space, formed by all the species that are part of a community (Cornwell et al. 2006); it does not consider species abundances. Functional richness was standardized by its maximum value, ranging between 0 and 1. Functional diversity was represented as the Rao quadratic index, which measures the pairwise distance between species in a distance matrix of all the species in a community, accounting for their abundances (Botta-Dukát 2005). Finally, we calculated functional uniqueness as the difference between the Simpson index and the Rao index, as proposed by Ricotta et al. (2016). This index measures the degree to which a community is composed of species with unique or redundant traits (Ricotta et al. 2016).
Measures of the functional structure of communities are inherently correlated with species richness; therefore, we obtained standardized effects sizes (SES) of functional richness, functional diversity, and functional uniqueness. We created 999 null models by randomly swapping the names of the species in the matrix containing the functional traits of the species. We calculated the different functional metrics in each of the null communities. The SES values were then obtained by resting the mean of the null index from the observed value of the index, divided by the standard deviation of the null values.
To explore how the different habitat types influenced the functional composition of flocks, we calculated the community weighted mean (CWM) of each functional trait. This method assesses how the mean of a functional trait, weighted by the abundance of the different species in a flock, varied according to the different habitat types. All the indices and CWM were calculated using flock level as the unit of analysis.
We then explored the influence of habitat type on the different flock descriptors using linear mixed models. SES functional richness, SES functional diversity, and SES functional uniqueness of flocks were used as response variables and habitat type as a fixed factor. To assess differences in the functional composition of flocks among habitat types, we constructed linear mixed models using the CWMs of each functional trait (body mass, bill index, HWI, tail index, and tarsus index) and habitat type as response variables. In all of the described models, we nested flocks observed on the same transect and during the same sampling period as the random components of the models. Statistical significance in the models was related to ANOVA tests with the Satterthwaite method (Kuznetsova et al. 2017). Post-hoc pairwise differences among habitat types were estimated by least square means, using P values of less than 0.05 to determine significant differences. For functional diversity indices and CWMs, we used the FD package in R (Laliberté and Legendre 2010).
RESULTS
Taxonomic structure of flocks
A total of 2,717 individuals from 43 species and 13 families of birds (taxonomic index given in Table S2) were recorded participating in 354 mixed species flocks in and around Cajas National Park (CNP) in Ecuador. Of the recorded species, 25.6% and 23.3% of species were New World flycatchers (Tyrannidae) and tanagers (Thraupidae), respectively. We found 126 flocks in the native forest of Mazán, 115 flocks in the regenerating forest of Llaviuco, and 113 flocks in the non-native forest of Sayausí. All habitat types had flocks of similar sizes (F2,47.2)=0.26, P=0.77; Fig. S1), and 79.9% of all flocks had 10 or fewer participants. Neither the species richness (X23=1.94, P=0.37; Fig. 3A, Fig. S2) nor species diversity (F2,52.9=0.71, P=0.49; Fig. 3B) of flocks were different across habitat types.
The five most frequent participants in all observed flocks regardless of habitat type (Table 2) were the Spectacled Redstart (Myioborus melanocephalus), Superciliaried Hemispingus (Thlypopsis superciliaris), Masked Flowerpiercer (Diglossa cyanea), Black-crested Warbler (Myiothlypis nigrocristata), and Black Flowerpiercer (Diglossa humeralis); out of 354 total flocks, 95.8% had at least one of these species present. The Spectacled Redstart, Superciliaried Hemispingus, and Masked Flowerpiercer were the most common flock participants, observed in 74.9%, 49.4%, and 45.5% of all flocks, respectively. Spectacled Redstarts were consistently the most frequent flock participants in all habitat types: 66.7% of native, 82.6% of regenerating, and 76.1% of non-native forest flocks. Supercilaried Hemispingus were the second most frequent participants in native (54.0% of flocks) and regenerating (47.0% of flocks) forest, while in non-native forest these were Masked Flowerpiercers (55.8% of flocks). Black-crested Warblers and Black Flowerpiercers were observed in 38.1% and 35.0% of all flocks, respectively, although their flock frequencies relative to those of other species in each habitat type varied.
The CCA indicated a significant effect of habitat type on flock species composition (CCA 1: F1=4.76, P<0.01; CCA 2: F1=3.37, P<0.01); however, the amount of variance explained by the first two canonical axes was low, indicating that there are other factors explaining a larger proportion of the changes in species composition among flocks (Fig. 4). All species scores can be found in Supplemental Table S3. The first CCA showed high scores for both native forest and regenerating forests (Fig. 4), represented by species that included three species of Chat-Tyrants (Crowned, Ochthoeca frontalis; , Brown-backed, Ochthoeca fumicolor; Rufous-breasted, Ochthoeca rufipectoralis), White-banded Tyrannulet (Mecocerculus stictopterus), and (Conirostrum sitticolor). Flocks found in the non-native forest had lower values on the first CCA (Fig. 4) and were represented by the White-browed Spinetail (Hellmayrea gularis), Plushcap (Catamblyrhynchus diadema), White-Crested Elaenia (Elaenia albiceps), and Azara’s Spinetail (Synallaxis azarae). The second CCA depicted flocks in the regenerating forest with positive values (Fig. 4), represented by species such as the Rufous-collared Sparrow (Zonotrichia capensis), Sedge Wren (Cistothorus platensis), and Blue-backed Conebill (Conirostrum sitticolor), and regenerating forest and non-native forest had low values of this CCA (Fig. 4) represented by the Rufous-breasted Chat-Tyrant, Tawny-rumped Tyrannulet (Phyllomyias uropygialis), and Black-capped Tyrannulet (Phyllomyias nigrocapillus).
Functional structure of flocks
Functional richness was not different among the three habitat types (F=0.66, P=0.52; Fig. 5A), while there were significant differences in functional diversity (F=4.60, P=0.01; Fig. 5B) and functional uniqueness (F=4.53, P=0.01; Fig. 5C). Regenerating forest showed flocks with higher values of functional diversity and functional uniqueness than flocks in native and non-native forest (Fig. 5).
Community weighted means (CWM) of several functional traits of flocks differed significantly among habitat types (Fig. 6). Flocks in native and regenerating forests presented higher mean body mass (F2,51.2=4.77, P=0.01) and bill index (F2,45.01=3.60, P=0.03) than flocks in non-native forest (Fig. 6A, B). HWI was similar among habitat types (F2,35.1=1.79, P=0.16; Fig. 6C). Moreover, flocks in native forest had smaller tarsus (F2,54.3=3.34, P=0.04) and tail indices (F2,51.85=3.01, P=0.05) than flocks in the other habitats (Fig. 6D, E).
DISCUSSION
Contrary to our expectations, we found few impacts of land use change on the taxonomic and functional structures of mixed species flocks in the tropical Andes of southern Ecuador. Flocks in native forest, forest regenerating from historical use as pastureland, and non-native forest composed of Eucalyptus sp. and, to a lesser extent, Pinus radiata showed no differences in species richness, species diversity, or functional richness of flocks. We did find influences of native vegetation on flock species composition, although habitat type explained a low proportion of the variation and other unknown factors might have larger impacts on these changes in composition. Functional diversity and uniqueness were significantly greater in regenerating forest flocks, while flocks in native and non-native forests showed no differences in any functional structure metrics. Lastly, we found significant differences in all functional trait indices except HWI, suggesting that while the total variety of traits (i.e., diversity) in flocks did not vary across different sites, habitat type still affected individual functional traits likely due to differences in vegetation composition and structure.
While numerous studies have examined impacts of anthropogenic activities on the taxonomic structure of mixed species flocks (e.g., Lee et al. 2005, Knowlton and Graham 2011, Goodale et al. 2015, Montaño-Centellas and Jones 2021), few have looked at responses of functional structure of flocks to the same activities (Jones and Robinson 2020) and to changing environmental conditions (Zhang et al. 2020, Dri et al. 2022). While species and functional diversities are related, they do not always respond similarly to disturbances and only looking at one or the other may obscure the magnitude of habitat disturbance impacts to mixed species flocks (Cadotte et al. 2011). For example, assemblages with functionally unique species (or species with no overlapping traits) may experience losses in functional diversity with no change in species richness as species that are lost are replaced by species that arrive in the community (Fonseca and Ganade 2001, Mayfield et al. 2010). This helps to explain our results, as we found no changes to species richness and diversity or to the functional richness of flocks, but there were significant differences in the functional diversity and uniqueness of flocks among the native, regenerating, and non-native forests.
Taxonomic structure of flocks
Previous studies found smaller and less speciose flocks in disturbed habitats with altered vegetation (Lee et al. 2005, Knowlton and Graham 2011, Jones and Robinson 2020, Montaño-Centellas and Jones 2021), but our results showed no effect of habitat type on the size, species richness, or species diversity of flocks. This may be explained by the reliable presence of five nuclear flocking species (Spectacled Redstart, Superciliaried Hemispingus, Masked Flowerpiercer, Black-crested Warbler, and Black Flowerpiercer), identified based on their high connectivities to satellite species and frequent participation in flocks (Vasquez-Ávila et al. 2021). Studies done on mixed flocks in other regions in the Andes have also identified various tanagers (Thraupidae) as nuclear species and have found similar frequent participation in flocks by many of the same or closely related species that we observed (Remsen et al. 1985, Bohórquez 2003, Arbeláez-Cortés et al. 2011). Nuclear individuals initiate flock formation and can maintain cohesion even in disturbed landscapes (Mammides et al. 2015), and 95.8% of all observed flocks had at least one participating nuclear individual. These species are abundant across the region, have low- to mid-sensitivities to disturbance, and are either intermediate habitat specialists or generalists (Parker et al. 1996, Tinoco et al. 2019). Taken altogether, it seems that these five nuclear species are relatively unaffected by land use change in our study area and their reliable presence and redundancy in flocks can potentially explain the homogenized flock size and richness observed across habitat types (Maldonado-Coelho and Marini 2004, Mammides et al. 2015, Jones and Robinson 2020, Jones and Robinson 2021).
In contrast, species composition of flocks was significantly, albeit weakly, affected by habitat type, and our results indicate other factors have stronger influences on these compositional changes. New World flycatchers (Tyrannidae) and, to a lesser extent, tanagers (Thraupidae) and ovenbirds (Furnariidae) represented the species with the greatest turnover in flock composition across habitat types. Our results are in line with those from other studies that show similar replacement of forest-interior New World flycatchers and ovenbirds with more generalist tanagers (Jones and Robinson 2020) and compositions being influenced by vegetation complexity (Montaño-Centellas and Jones 2021) in Andean flocks. Changes in species composition in flocks did not coincide with differences in flock size, species richness, or species diversity, indicating that as species are lost from flocks in one habitat they are readily replaced by another.
Functional structure of flocks
Our findings indicate that regenerating forest flocks had greater functional diversities and functional uniqueness than flocks in native or non-native forest. Vegetation composition and structural diversity of each habitat type likely explain these differences in functional metrics. Non-native forest was primarily composed of Eucalyptus sp. and Pinus radiata trees in strips of old windbreaks surrounded by pasture and agricultural land, and these structurally simple monoculture stands were relatively homogeneous. In comparison, native forest possessed a greater diversity of native vegetation and a dense forest structure, but the functional diversity and uniqueness of flocks were unexpectedly similar to that of non-native forest flocks. The greatest diversity of vegetation composition and structure was found in the regenerating forest; with high habitat heterogeneity and structural complexity, this habitat contains more microhabitats and more available niches, and thus can support a greater diversity of functional traits and both generalized and specialized species (MacArthur and MacArthur 1961, Zhang et al. 2013). Andean flocks have an open-membership nature with species joining and leaving as flocks pass through their territory (Poulsen 1996, Jones and Robinson 2020), and compositions may change from forest-specialists to generalists as flocks move into disturbed areas (Zhang et al. 2013, Valiente-Banuet et al. 2015). While we showed changes to flock composition across habitat types, the trait turnover between native and non-native forest flocks is not strong enough to demonstrate significant changes in these two functional metrics.
Interestingly, functional richness of flocks was the only functional metric that did not differ across habitat types. Dri et al. (2022) similarly found no effect of habitat type on functional richness of flocks after controlling for size differences. Meanwhile, Jones and Robinson (2020) found a significant difference in flocks’ functional richness depending on the density of large diameter old-growth trees, but we found no increase in richness for native forest flocks. Smaller flocks such as ours may show less variability in functional traits as compared to larger flocks, although habitat type still affects the clustering or overdispersion of certain traits (Dri et al. 2022). As with species richness, reliable nuclear species and maintenance of cohesive flocks can also lead to homogenized functional richness due to specialized species persisting in disturbed habitats via facilitative interactions (Morse 1977, Mammides et al. 2015). When species abundance is considered, however, the functional diversity and uniqueness of flocks can concurrently show changes across habitat types, in line with our results.
Flocks in native and regenerating forests contained heavier species and/or more large-bodied individuals than flocks in non-native forest. Mixed species flocks are typically composed of smaller-bodied birds (Sainz-Borgo et al. 2018, Zhang et al. 2020), but larger species may join as well to benefit from the interspecific interactions. Several of the heaviest flocking species observed (Grey-breasted Mountain Toucan, Andigena hypoglauca; Chestnut-crowned Antpitta, Grallaria ruficapilla; Red-crested Cotinga, Ampelion rubrocristatus; and Masked Trogon, Trogon personatus) were present in native and/or regenerating forest flocks but absent from non-native forest flocks. This may be due to having higher sensitivities to disturbance (Parker et al. 1996), as larger-bodied species are typically more sensitive to habitat alterations (Blumstein et al. 2005, Srinivasan 2013). Another possible explanation for this trend in body mass may be varying predation risks depending on the habitat. As compared to native and regenerating forests, the non-native forest was more open with a simplified vegetation structure (Vasquez-Ávila et al. 2021). Birds may experience greater predation rates in habitats with less cover and less structural complexity (Rodríguez et al. 2001), and so smaller-bodied individuals might join flocks more frequently in non-native forest as predator escape can be maximized when all participants are similarly sized (Goodale et al. 2010, Dri et al. 2022).
Similar to body mass, flocks in native and regenerating forests also showed higher bill indices than flocks in non-native forest. Bill morphology is affected by foraging substrate and food availability, with species often adapting to a specific food resource and foraging guild (Grant and Grant 1996, Friedman et al. 2019). In comparison to native forest, non-native eucalyptus forest with a homogenized vegetation structure often provides insufficient food resources, which can affect the presence of certain foraging guilds (Majer and Recher 1999). Results from previous studies suggest imported eucalyptus vegetation has reduced arthropod abundance and diversity, especially in stands without a developed understory, leading to fewer specialist insectivorous species (Calviño-Cancela 2013, Jacoboski et al. 2016). Insufficient food resources could explain the trait turnover we found in bill index, with species having on average longer, narrower bills in non-native forest flocks than those in other habitats. The simplified structural complexity and lower diversity of non-native forest vegetation may also contribute to trait turnover, as mixed flocks in other regions have also shown negative relationships between bill length and shrub diversity (Zhang et al. 2020). Flycatchers, which typically have wider, shorter bills and a higher bill index due to their aerial foraging strategy (Kennedy et al. 2019), were more frequently seen in native and regenerating forest flocks; moreover, several flycatcher species were not found at all in non-native forest flocks. While we did not find significant changes in species richness or diversity of flocks across the three habitats, there is still trait turnover occurring as several specialized and/or sensitive species are being lost from flocks, a result of alterations to native vegetation.
The flocks in native forest had significantly shorter tails and tarsi than in regenerating or non-native forest. Tail and tarsus length are functional traits closely associated with movement; tail length is related to maneuverability and agility when flying, and tarsus length is associated with stability when perching and locomotion (Proctor and Lynch 1993, Thomas and Balmford 1995, Zeffer et al. 2003). This implies that different habitats should have species with tails and tarsi that are best suited to moving around in the local environment (Miles and Ricklefs 1984, Kennedy et al. 2019, Dri et al. 2022). Longer tails typically increase maneuverability and can aid in agility, as in aerial hawkers; however, longer tails with greater areas also increase drag, aid in climbing trees (particularly those with rough bark and more friction) and, are more susceptible to damage via collisions with branches or vegetation (Norberg 1979, Norberg 1986, Thomas and Balmford 1995). Shorter tails provide more stability when clinging to trees (particularly those with smooth bark and less friction), typically decrease drag, and are less likely to collide with vegetation in cluttered environments (Norberg 1986, Thomas 1997). As a result, tail length faces many selection pressures from habitat and foraging strategies and many trade-offs exist, which makes explaining the patterns of tail length in our mixed flocks difficult. Native forest in CNP is a relatively dense and cluttered environment, and the tails of bird species here may face selection pressures away from flight efficiency towards clinging to vegetation and lower risks of damage via collisions. In the future, tail shape and area should be added to or used instead of tail length, as these traits consider the lift-to-drag and moment-to-drag ratios (Thomas 1977, Balmford et al. 1993) and so are better proxies for flight efficiency and movement.
Tarsus length also has trade-offs regarding movement through habitat and foraging strategy. Shorter tarsi are lighter and provide increased stability when clinging to and climbing trees or when perching on and hanging from branches, while longer tarsi are heavier and increase both step length and locomotion speed on the ground (Norberg 1979, Zeffer et al. 2003). The shorter tarsi found in mixed flocks in native forest as compared to regenerating or non-native forests is likely driven by the Furnariidae species, which were found most frequently in native forest flocks. Furnariidae woodcreepers had some of the shortest tarsi index values, particularly the Flammulated Treehunter (Thripadectes flammulatus) and Streaked Tuftedcheek (Pseudocolaptes boissonneautii), due to their need for stability when climbing trees (Norberg 1979, Zeffer et al. 2003).
The HWI was the only functional trait with no significant differences across habitat types, and all flocks showed relatively low to moderate HWI values. This measurement is used as a proxy for flight efficiency and is associated with dispersal ability, foraging strategy, and flight style (Rayner 1988, Claramunt et al. 2012, Sheard et al. 2020. Bird species that participated in flocks were predominantly understory or midstory species foraging in denser habitats (Parker et al. 1996). This foraging strategy and stratum hinge on maneuverability, and thus we can expect these species to have lower HWI (Norberg 1979, Claramunt 2021). Therefore, this suggests that the high-elevation study area has a greater potential to influence flocking species’ wing morphology rather than land use change via filtering out upper canopy foragers that may otherwise participate in mixed species flocks and reveal changes in the HWI.
CONCLUSION
In summary, we found land use change to have minimal effects on the taxonomic and functional structures of mixed species flocks in and around CNP, which indicates flocking as a potential mechanism for maintaining both species and functional diversities in areas with anthropogenic activities. The only aspects of flocks that were impacted by land use change were the species composition, functional diversity, and functional uniqueness. However, the impacts to each were either minimal (species composition) or showed no differences (functional diversity and uniqueness) between pristine native forest and non-native forest with extensive anthropogenic land use change. We also found habitat type to have a filtering effect on four of five functional traits found in flocks, demonstrating land use change to still have a background effect on functional metrics of flocks across all sites.
Our study had several limitations mainly tied to the nature of our mixed species flocks. As Andean flocks have open memberships with species joining and leaving as flocks pass through their territory, the same individuals may have been counted in more than one flock. We tried to account for these potential pseudoreplication issues by including flocks found on the same transect and in the same sampling period as random factors in our models. In addition, our results may not be applicable to other areas and at lower elevations, due to the small size of our flocks. Larger, more speciose flocks may show more significant impacts of land use change simply due to more participating species and individuals, some of which may have higher sensitivities to disturbance and possess more unique traits. Lastly, while we found that flock taxonomic and functional structures were generally maintained among different land uses, specifically between native and non-native forest, we did not evaluate the same metrics for the general avian community in these habitats. In the future, we recommend including additional analyses to ascertain potential differences between taxonomic and functional structures of non-flocking and flocking species to confirm if participation in mixed species flocks is mitigating impacts of land use change to avian species in and around CNP.
Our study adds to the literature on anthropogenic impacts on avian communities and mixed species flocks. Few studies to date have incorporated functional diversity metrics in mixed species flock analyses (Jones and Robinson 2020, Zhang et al. 2020, Dri et al. 2022) and none have looked specifically at effects of land use change. As functional diversity has become the focus of many studies evaluating direct effects of anthropogenic activities on biodiversity, on communities of varying taxa, and on ecosystem stability and functioning, our study contributes to this body of knowledge. We conclude that mixed species flocks and the nuclear species should be the focus of management and conservation decisions to maintain all aspects of avian diversity in the face of increasing and widespread anthropogenic disturbances to landscapes. By protecting flocking dynamics and the interspecific facilitations they provide, managers can potentially maintain sensitive or threatened species and ecosystem services provided by birds even in highly disturbed areas.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.
ACKNOWLEDGMENTS
We acknowledge Jeremiah Trimble and the Harvard Museum of Comparative Zoology for allowing access to their extensive avian collection. Travel to Cajas National Park in July 2019 was funded by the Davis International Fellowship, awarded by Wheaton College (MA), to assist in the yearly mist-netting campaign run and funded by the Universidad del Azuay. Field data collection was supported with a research grant from SENESCYT, under the project “Respuesta espacio-temporales de las comunidades de aves y murciélagos a gradientes altitudinales y de perturbación en tres ecosistemas al sur del Ecuador”. BTR and BV were also funded by Vicerrectorado de Investigaciones, Project number 2018-0092, 2019-0095.
DATA AVAILABILITY
The data that support the findings of this study are openly available in Open Science Framework at https://doi.org/10.17605/OSF.IO/PUXFZ.
LITERATURE CITED
Arbeláez-Cortés, E., H. A. Rodríguez-Correa, and M. Restrepo-Chica. 2011. Mixed bird flocks: patterns of activity and species composition in a region of the Central Andes of Colombia. Revista Mexicana de Biodiversidad 82:639-651. https://doi.org/10.22201/ib.20078706e.2011.2.468
Balmford, A. B., A. L. R. Thomas, and I. L. Jones. 1993. Aerodynamics and the evolution of long tails in birds. Letters to Nature 361:628-631. https://doi.org/10.1038/361628a0
Birdlife International. 2021. IUCN Red List for birds. BirdLife Global Office, Cambridge, UK. https://www.birdlife.org (10 May 2021).
Blumstein, D. T., E. Fernández-Juricic, P. A. Zollner, and S. C. Garity. 2005. Inter-specific variation in avian responses to human disturbance. Journal of Applied Ecology 42:943-953. https://doi.org/10.1111/j.1365-2664.2005.01071.x
Bohórquez, C. I. 2003. Mixed-species bird flocks in a montane cloud forest of Colombia. Ornitologia Neotropical 14:67-78.
Borah, B., S. Quader, and U. Srinivasan. 2018. Responses of interspecific associations in mixed-species bird flocks to selective logging. Journal of Applied Ecology 55:1637-1646. https://doi.org/10.1111/1365-2664.13097
Botta-Dukát, Z. 2005. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. Journal of Vegetation Science 16(5):533-540. https://doi.org/10.1111/j.1654-1103.2005.tb02393.x
Cadotte, M. W., K. Carscadden, and N. Mirotchnick. 2011. Beyond species: functional diversity and the maintenance of ecological processes and services. Journal of Applied Ecology 48:1079-1087. https://doi.org/10.1111/j.1365-2664.2011.02048.x
Calviño-Cancela, M. 2013. Effectiveness of eucalypt plantations as a surrogate habitat for birds. Forest Ecology and Management 310:692-699. https://doi.org/10.1016/j.foreco.2013.09.014
Cardinale, B. J., J. E. Duffy, A. Gonzalez, D. U. Hooper, C. Perrings, P. Venail, A. Narwani, G. M. Mace, D. Tilman, D. A. Wardle, A. P. Kinzig, G. C. Daily, M. Loreau, J. B. Grace, A. Larigauderie, D. S. Srivastava, and S. Naeem. 2012. Biodiversity loss and its impact on humanity. Nature 486:59-67. https://doi.org/10.1038/nature11148
Celleri, R., P. Willems, W. Buytaert, and J. Feyen. 2007. Space-time rainfall variability in the Paute Basin, Ecuadorian Andes. Hydrological Processes 21:3316-3327. https://doi.org/10.1002/hyp.6575
Chacón-Vintimilla, G. 2016. Mazán y Llaviuco: 40 años después. UDA AKADEM 1:30-37. https://doi.org/10.33324/udaakadem.vi1.116 (15 May 2021).
Claramunt, S. 2021. Flight efficiency explains differences in natal dispersal distances in birds. Ecology 102(9):e03442. https://doi.org/10.1002/ecy.3442
Claramunt, S. E. P. Derryberry, J. V. Remsen Jr., and R. T. Brumfield. 2012. High dispersal ability inhibits speciation in a continental radiation of passerine birds. Proceedings of the Royal Society B 279:1567-1574. https://doi.org/10.1098/rspb.2011.1922
Clements, H. S., A. De Vos, J. C. Bezerra, K. Coetzer, K. Maciejewski, P. J. Mograbi, and C. Shackleton. 2021. The relevance of ecosystem services to land reform policies: insights from South Africa. Land Use Policy 100:104939. https://doi.org/10.1016/j.landusepol.2020.104939
Coetzee, B. W. T., and S. L. Chown. 2016. Land use change promotes avian diversity at the expense of species with unique traits. Ecology and Evolution 6(21):7610-7622. https://doi.org/10.1002/ece3.2389
Colorado, G. J., and A. D. Rodewald. 2015. Response of mixed-species flocks to habitat alteration and deforestation in the Andes. Biological Conservation 188:72-81. https://doi.org/10.1016/j.biocon.2015.02.008
Cordero, P. 2002. Information sheet on Ramsar wetlands. Ramsar Convention on Wetlands of International Importance, Report EC1203RIS, Gland, Switzerland. https://rsis.ramsar.org/RISapp/files/RISrep/EC1203RISformer2002_EN.pdf (15 May 2021).
Cornwell, W. K., D. W. Schwilk, and D. D. Ackerly. 2006. A trait-based test for habitat filtering: convex hull volume. Ecology 87(6):1465-1471. https://doi.org/10.1890/0012-9658(2006)87[1465:ATTFHF]2.0.CO;2
Di Marco, M., T. D. Harwood, A. J. Hoskins, C. Ware, S. L. L. Hill, and S. Ferrier. 2019. Projecting impacts of global climate and land use scenarios on plant biodiversity using compositional-turnover modelling. Global Change Biology 25(8):2763-2778. https://doi.org/10.1111/gcb.14663
Díaz, S., and M. Cabido. 2001. Vive la difference: plant functional diversity matters to ecosystem processes. Trends in Ecology and Evolution 16(11):646-655. https://doi.org/10.1016/S0169-5347(01)02283-2
Díaz, S., A. Purvis, J. H. Cornelissen, G. M. Mace, M. J. Donoghue, R. M. Ewers, P. Jordano, and W. D. Pearse. 2013. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecology and Evolution 3(9):2958-2975. https://doi.org/10.1002/ece3.601
Díaz, S., J. Settele, E. S. Brondízio, H. T. Ngo, J. Agard, A. Arneth, P. Balvanera, K. A. Brauman, S. H. M. Butchart, K. M. A. Chan, L. A. Garibaldi, K. Ichii, J. Liu, S. M. Subramanian, G. F. Midgley, P. Miloslavich, Z. Molnár, D. Obura, A. Pfaff, S. Polasky, A. Purvis, J. Razzaque, B. Reyers, R. R. Chowdhury, Y.-J. Shin, I. Visseren-Hamakers, K. J. Willis, and C. N. Zayas. 2019. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366(6471):eaax3100. https://doi.org/10.1126/science.aax3100
Dri, G. F., N. C. Cáceres, F. Della-Flora, and C. S. Dambros. 2022. Mixed-species bird flocks enhance the benefits of group aggregation by minimizing variation in body mass while maximizing variation in diet. Oikos:e09115. https://doi.org/10.1111/oik.09115
ETAPA. 2018. Actualización del plan de manejo del Parque Nacional Cajas: documento final. Empresa de Telecomunicaciones, Agua Potable, Alcantarillado y Saneamiento, Cuenca, Ecuador. https://www.ambiente.gob.ec/wp-content/uploads/downloads/2018/03/ACUERDO-001-ANEXO-PAQUE-NACIONAL-CAJAS.pdf (15 May 2021).
Fonseca, C. R., and G. Ganade. 2001. Species functional redundancy, random extinctions and the stability of ecosystems. Journal of Ecology 89:118-125. https://doi.org/10.1046/j.1365-2745.2001.00528.x
Friedman, N. R., E. T. Miller, J. R. Ball, H. Kasuga, V. Remeš, and E. P. Economo. 2019. Evolution of a multifunctional trait: shared effects of foraging ecology and thermoregulation on beak morphology, with consequences for song evolution. Proceedings of the Royal Society B 286:20192474. https://doi.org/10.1098/rspb.2019.2474
Gardner, T. A., J. Barlow, I. S. Araujo, T. C. Ávila-Pires, A. B. Bonaldo, J. E. Costa, M. C. Esposito, L. V. Ferreira, J. Hawes, M. I. M. Hernandez, M. S. Hoogmoed, R. N. Leite, N. F. Lo-Man-Hung, J. R. Malcolm, M. B. Martins, L. A. M. Mestre, R. Miranda-Santos, W. L Overal, L. Parry, S. L. Peters, M. A. Ribeiro-Junior, M. N. F. Da Silva, C. Da Silva Motta, qnd C. A. Peres. 2008. The cost-effectiveness of biodiversity surveys in tropical forests. Ecology Letters 11(2):139-150. https://doi.org/10.1111/j.1461-0248.2007.01133.x
Gaston, K. J., T. M. Blackburn, and K. K. Goldewijk. 2003. Habitat conversion and global avian biodiversity loss. Proceedings of the Royal Society B 270:1293-1300. https://doi.org/10.1098/rspb.2002.2303
Goodale, E., and S. W. Kotagama. 2005. Testing the roles of species in mixed-species bird flocks of a Sri Lankan rainforest. Journal of Tropical Ecology 21(6):669-676. https://doi.org/10.1017/S0266467405002609
Goodale, E., G. Beauchamp, R. D. Magrath, J. C. Nieh, and G. D. Ruxton. 2010. Interspecific information transfer influences animal community structure. Trends in Ecology and Evolution 25(6):354-361. https://doi.org/10.1016/j.tree.2010.01.002
Goodale, E., P. Ding, X. Liu, A. Martínez, X. Si, M. Walters, and S. K. Robinson. 2015. The structure of mixed-species bird flocks, and their response to anthropogenic disturbance, with special reference to East Asia. Avian Research 6(8):14-24. https://doi.org/10.1186/s40657-015-0023-0
Grant, B. R., and P. R. Grant. 1996. High survival of Darwin’s finch hybrids: effects of beak morphology and diets. Ecology 77(2):500-509. https://doi.org/10.2307/2265625
Harrison, N. M., and M. J. Whitehouse. 2011. Mixed-species flocks: an example of niche construction? Animal Behavior 81:675-682. https://doi.org/10.1016/j.anbehav.2011.01.013
Hellmayr, C. E. 1925. Catalogue of birds of the Americas and related islands, part IV. Field Museum of Natural History, Chicago, IL, USA.
Hutto, R. L. 1987. A description of mixed-species insectivorous bird flocks in western Mexico. Condor 89:282-292. https://doi.org/10.2307/1368481
Hutto, R. L. 1994. The composition and social organization of mixed-species flocks in a tropical deciduous forest in western Mexico. Condor 96(1):105-118. https://doi.org/10.2307/1369068
Jacoboski, L. I., V. J. Debastiani, A. de Mendonça-Lima, and S. M. Hartz. 2016. How do diversity and functional nestedness of bird communities respond to changes in the landscape caused by eucalyptus plantations? Community Ecology 17(1):107-113. https://doi.org/10.1556/168.2016.17.1.13
Järvinen, O., and R. A. Väisänen. 1979. Changes in bird populations as criteria of environmental changes. Holarctic Ecology 2(2):75-80. https://doi.org/10.1111/j.1600-0587.1979.tb00684.x
Jones, H. H., and S. K. Robinson. 2020. Patch size and vegetation structure drive changes to mixed-species flock diversity and composition across a gradient of fragment sizes in the Western Andes of Colombia. Condor 122(2):duaa006. https://doi.org/10.1093/condor/duaa006
Jones, H. H., and S. K. Robinson. 2021. Vegetation structure drives mixed-species flock interaction strength and nuclear species roles. Behavioral Ecology 32(1):69-81. https://doi.org/10.1093/beheco/araa103
Jullien, M., and J. Clobert. 2000. The survival value of flocking in neotropical birds: reality or fiction? Ecology 81(12):3416-3430. https://doi.org/10.1890/0012-9658(2000)081[3416:TSVOFI]2.0.CO;2
Kennedy, J. D., P. Z. Marki, J., Fjeldså, and C. Rahbek. 2019. The association between morphological and ecological characters across a global passerine radiation. Journal of Animal Ecology 89(4):1094-1108. https://doi.org/10.1111/1365-2656.13169
Knowlton, J. L., and C. H. Graham. 2011. Species interactions are disrupted by habitat degradation in the highly threatened Tumbesian region of Ecuador. Ecological Applications 21(8): 2974-2986. https://doi.org/10.1890/10-1886.1
Kraft, N. J. B., and D. D. Ackerly. 2010. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecological Monographs 80(3):401-422. https://doi.org/10.1890/09-1672.1
Kuznetsova, A., P. B. Brockhoff, and R. H. B. Christensen. 2017. Imer Test package: test in linear mixed effects models. Journal of Statistical Software 82:13. https://doi.org/10.18637/jss.v082.i13
Laliberté, E., and P. Legendre. 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299-305. https://doi.org/10.1890/08-2244.1
Latta, S. C., and J. M. Wunderle Jr. 1996. The composition and foraging ecology of mixed-species flocks in pine forests of Hispaniola. Condor 98(3):595-607. https://doi.org/10.2307/1369572
Latta, S. C., B. A. Tinoco, P. X. Astudillo, and C. H. Graham. 2011. Patterns and magnitude of temporal change in avian communities in the Ecuadorian Andes. Condor 113(1): 24-40. https://doi.org/10.1525/cond.2011.090252
Lee, T. M., M. C. K. Soh, N. Sodhi, L. P. Koh, and S. L.-H. Lim. 2005. Effects of habitat disturbance on mixed species bird flocks in a tropical sub-montane rainforest. Biological Conservation 122:193-204. https://doi.org/10.1016/j.biocon.2004.07.005
Lewis, H. M., R. Law, and A. J. McKane. 2008. Abundance-body size relationships: the roles of metabolism and population dynamics. Journal of Animal Ecology 77:1056-1062. https://doi.org/10.1111/j.1365-2656.2008.01405.x
MacArthur, R. H., and J. W. MacArthur. 1961. On bird species diversity. Ecology 42(3):594-598. https://doi.org/10.2307/1932254
Majer, J. D., and H. F. Racher. 1999. Are eucalypts Brazil’s friend or foe? An entomological viewpoint. Anais da Sociedade Entomológica do Brasil 28(2):185-200. https://doi.org/10.1590/S0301-80591999000200001
Maldonado-Coelho, M., and M. Â. Marini. 2004. Mixed-species bird flocks from Brazilian Atlantic forest: the effects of forest fragmentation and seasonality on their size, richness and stability. Biological Conservation 116:19-26. https://doi.org/10.1016/S0006-3207(03)00169-1
Mammides, C., J. Chen, U. M. Goodale, S. W. Kotagama, S. Sidhu, and E. Goodale. 2015. Does mixed-species flocking influence how birds respond to a gradient of land use intensity? Proceedings of the Royal Society B 282:20151118. https://doi.org/10.1098/rspb.2015.1118
Mason, N. W. H., D. Mouillot, W. G. Lee, and J. B. Wilson. 2005. Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos 111:112-118. https://doi.org/10.1111/j.0030-1299.2005.13886.x
Matthysen, E., F. Collet, and J. Cahill. 2008. Mixed flock composition and foraging behavior of insectivorous birds in undisturbed and disturbed fragments of high-Andean polylepis woodland. Ornitologia Neotropical 19:403-416.
Mayfield, M. M., S. P. Bonser, J. W. Morgan, I. Aubin, S. McNamara, and P. A. Vesk. 2010. What does species richness tell us about functional trait diversity? Predictions and evidence for responses of species and functional trait diversity to land use change. Global Ecology and Biogeography 19:423-431. https://doi.org/10.1111/j.1466-8238.2010.00532.x
Miles, D. B., and R. E. Ricklefs. 1984. The correlation between ecology and morphology in deciduous forest passerine birds. Ecology 65(5):1629-1640. https://doi.org/10.2307/1939141
Minga, D. 2000. Árboles y arbustos del bosque de Mazán tomo II. Empresa Pública Municipal de Telecomunicaciones, Agua Potable y Alcantarillado, Cuenca, Ecuador.
Montaño-Centellas, F. A. and H. H. Jones. 2021. Temperature and vegetation complexity structure mixed-species flocks along a gradient of elevation in the tropical Andes. Ornithology 138:1-18. https://doi.org/10.1093/ornithology/ukab027
Morse, D. H. 1970. Ecological aspects of some mixed-species foraging flocks of birds. Ecological Monographs 40(1):119-168. https://doi.org/10.2307/1942443
Morse, D. H. 1977. Feeding behavior and predator avoidance in heterospecific groups. BioScience 27(5):332-339. https://doi.org/10.2307/1297632
Norberg, U. M. 1979. Morphology of the wings, legs and tail of three coniferous forest tits, the goldcrest, and the treecreeper in relation to locomotor pattern and feeding station selection. Philosophical Transactions of the Royal Society B 287:131-165. https://doi.org/10.1098/rstb.1979.0054
Norberg, R. Å. 1986. Treecreeper climbing: mechanics, energetics, and structural adaptations. Ornis Scandinavica 17(3):191-209. https://doi.org/10.2307/3676828
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. Minchin, B. O´Hara, G. Simpson, P. Solymos, M. H. Stevens, E. Szoecs, and H. Wagner. 2020. Vegan: community ecology package. R package version 2.5-7.
Parker, T. A., III, D. F. Stotz, and J. W. Fitzpatrick. 1996. Ecological and distributional databases. In: Neotropical birds: ecology and conservation (D. F., Stotz, J. W. Fitzpatrick, T. A. Parker III, and D. K. Moskovits, eds), pp. 113-436. University of Chicago Press, Chicago, IL, USA.
Pereira, H. M., and H. D. Cooper. 2006. Towards the global monitoring of biodiversity change. Trends in Ecology and Evolution 21(3):123-129. https://doi.org/10.1016/j.tree.2005.10.015
Pereira, H. M., L. M. Navarro, and I. S. Martins. 2012. Global biodiversity change: the bad, the good, and the unknown. Annual Review of Environment Resources 37:25-50. https://doi.org/10.1146/annurev-environ-042911-093511
Petchey, O. L., and K. J. Gaston. 2006. Functional diversity: back to basics and looking forward. Ecology Letters 9:741-758. https://doi.org/10.1111/j.1461-0248.2006.00924.x
Peters, R. H. 1983. The ecological implications of body size. Cambridge University Press, New York, NY, USA. https://doi.org/10.1017/CBO9780511608551
Pocock, M. J. O. 2011. Can traits predict species’ vulnerability? A test with farmland passerines in two continents. Proceedings of the Royal Society B 278(1711):1532-1538. https://doi.org/10.1098/rspb.2010.1971
Poulsen, B. O. 1996. Structure, dynamics, home range and activity pattern of mixed-species bird flocks in a montane alder-dominated secondary forest in Ecuador. Journal of Tropical Ecology 12(3): 333-343. https://doi.org/10.1017/S0266467400009524
Proctor, N. S., and P. J. Lynch. 1993. Manual of ornithology: avian structure and function. Yale University, New Haven, CT, USA.
R Development Core Team. 2021. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org.
Rayner, J. M. V. 1988. Form and function in avian flight. In: Current ornithology, vol 5 (R. F. Johnston, ed), pp. 1-66. Springer, Boston, MA, USA. https://doi.org/10.1007/978-1-4615-6787-5_1
Reiss, J., J. R. Bridle, J. M. Montoya, and G. Woodward. 2009. Emerging horizons in biodiversity and ecosystem functioning research. Trends in Ecology and Evolution 24(9):505-514. https://doi.org/10.1016/j.tree.2009.03.018
Remsen, J. V. Jr. 1985. Community organization and ecology of birds of high elevation humid forest of the Bolivian Andes. Ornithological Monographs 36:733-756. https://doi.org/10.2307/40168314
Ricotta, C., F. de Bello, M. Moretti, M. Caccianiga, B. E. L. Cerabolini, and S. Pavoine. 2016. Measuring the functional redundancy of biological communities: a quantitative guide. Methods Ecology and Evolution 7(11):1386-1395. https://doi.org/10.1111/2041-210X.12604
Rocha, J., R. R. Laps, C. G. Machado, and S. Campiolo. 2019. The conservation value of cacao agroforestry for bird functional diversity in tropical agricultural landscapes. Ecology and Evolution 9:7903-7913. https://doi.org/10.1002/ece3.5021
Rodríguez, A., H. Andrén, and G. Jansson. 2001. Habitat-mediated predation risk and decision making of small birds at forest edges. Oikos 95:383-396. https://doi.org/10.1034/j.1600-0706.2001.950303.x
Sainz-Borgo, C., S. Koffler, and K. Jaffe. 2018. On the adaptive characteristics of bird flocks: small birds form mixed flocks. Ornithología Neotropical 29:289-296.
Şekercioḡlu, C. H. 2006. Increasing awareness of avian ecological function. Trends in Ecology and Evolution 21(8):464-471. https://doi.org/10.1016/j.tree.2006.05.007
Şekercioḡlu, C. H. 2012. Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. Journal of Ornithology 153:153-161. https://doi.org/10.1007/s10336-012-0869-4
Sheard, C., M. H. C. Neate-Clegg, N. Alioravainen, S. E. I. Jones, C. Vincent, H. E. A. Macgregor, T. P. Bregman, S. Claramunt, and J. A. Tobias. 2020. Ecological drivers of global gradients in avian dispersal inferred from wing morphology. Nature Communications 11:2463. https://doi.org/10.1038/s41467-020-16313-6
Sridhar, H., G. Beauchamp, and K. Shanker. 2009. Why do birds participate in mixed-species foraging flocks? A large-scale synthesis. Animal Behaviour 78:337-347. https://doi.org/10.1016/j.anbehav.2009.05.008
Srinivasan, U. 2013. A slippery slope: logging alters mass-abundance scaling in ecological communities. Journal of Applied Ecology 50:920-928. https://doi.org/10.1111/1365-2664.12123
Ter Braak, C. J. F. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67(5):1167-1179. https://doi.org/10.2307/1938672
Thomas, A. L. R. 1997. On the tails of birds. BioScience 47(4):215-225.
Thomas, A. L. R., and A. Balmford. 1995. How natural selection shapes birds’ tails. The American Naturalist 146(6):848-868. https://doi.org/10.2307/1313075
Tilman, D. 2001. Functional diversity. In: Encyclopedia of biodiversity (S. A. Levin, ed), pp. 109-120. Academic Press, San Diego, CA, USA. https://doi.org/10.1016/B0-12-226865-2/00132-2
Tinoco, B. A., L. Graham, P. X. Astudillo, A. Nieto, J. M. Aguilar, S. C. Latta, and C. H. Graham. 2019. Survival estimates of bird species across altered habitats in the tropical Andes. Journal of Field Ornithology 90(2):105-116. https://doi.org/10.1111/jofo.12293
Valiente-Banuet, A., M. A. Aizen, J. M. Alcántara, J. Arroyo, A. Cocucci, M. Galetti, M. C. García, D. García, J. M. Gómez, P. Jordano, R. Medel, L. Navarro, J. R. Obeso, R. Oviedo, N. Ramírez, P. J. Rey, A. Traveset, M. Verdú, and R. Zamora. 2015. Beyond species loss: the extinction of ecological interactions in a changing world. Functional Ecology 29(3):299-307. https://doi.org/10.1111/1365-2435.12356
Vásquez-Ávila, B., J. L. Knowlton, C. I. Espinosa, and B. A. Tinoco. 2021. Habitat alteration modifies the structure and function of mixed-species flocks in an Andean landscape. Biotropica 53(4):1153-1162. https://doi.org/10.1111/btp.12957
Vitousek, P. M. 1997. Human domination of Earth’s ecosystems. Science 277(5325):494-499. https://doi.org/10.1126/science.277.5325.494
Walker, B., A. Kinzig, and J. Langridge. 1999. Plant attribute diversity, resilience, and ecosystem function: the nature and significance of dominant and minor species. Ecosystems 2(2):95-113. https://doi.org/10.1007/s100219900062
Weideman, E. A., J. A. Slingsby, R. L. Thomson, and B. T. W. Coetzee. 2020. Land cover change homogenizes functional and phylogenetic diversity within and among African savanna bird assemblages. Landscape Ecology 35(1):145-157. https://doi.org/10.1007/s10980-019-00939-z
Whelan, C. J., D. G. Wenny, and R. J. Marquis. 2008. Ecosystem services provided by birds. Annals of the New York Academy of Sciences 1134:25-60. https://doi.org/10.1196/annals.1439.003
Wilman, H., J. Belmaker, J. Simpson, C. De La Rosa, M. M. Rivadeneira, and W. Jetz. 2014. EltonTraits 1.0: species-level foraging attributes of the world’s birds and mammals. Ecology 95(7):2027. https://doi.org/10.1890/13-1917.1
Zeffer, A., L. C. Johansson, and Å. Marmebro. 2003. Functional correlation between habitat use and leg morphology in birds (Aves). Biological Journal of the Linnean Society 79(3):461-484. https://doi.org/10.1046/j.1095-8312.2003.00200.x
Zhang, Q., R. Han, Z. Huang, and F. Zou. 2013. Linking vegetation structure and bird organization: response of mixed-species bird flocks to forest succession in subtropical China. Biodiversity and Conservation 22(9):1965-1989. https://doi.org/10.1007/s10531-013-0521-5
Zhang, Q., R., M. Holyoak, E. Goodale, Z. Liu, Y. Shen, J. Liu, M. Zhang, A. Dong, and F. Zou. 2020. Trait-environment relationships differ between mixed-species flocking and nonflocking bird assemblages. Ecology 101(10):e03124. https://doi.org/10.1002/ecy.3124
Zou, F., H. Jones, G. J. Colorado Z., D. Jiang, T.-M. Lee, A. Martínez, K. Sieving, M. Zhang, Q. Zhang, and E. Goodale. 2018. The conservation implications of mixed-species flocking in terrestrial birds, a globally-distributed species interaction network. Biological Conservation 224:267-276. https://doi.org/10.1016/j.biocon.2018.06.004
Fig. 1

Fig. 1. Map of the study area including A) Sayausí, or non-native forest, B) Mazán, or native forest, and C) Llaviuco, or regenerating forest, valleys in and around Cajas National Park, Azuay province, Ecuador. White lines represent four 500 m transects used for flock observations. From Vásquez-Ávila et al. (2021).

Fig. 2

Fig. 2. Example of a mixed species flock found in the regenerating forest, composed of the species A) Streaked Tuftedcheek (Pseudocolaptes boissonneautii), B) Spectacled Redstart (Myioborus melanocephalus), C) Scarlet-bellied Mountain Tanager (Anisognathus igniventris), D) Black-crested Warbler (Myiothlypis nigrocristata), and E) Buff-breasted Mountain Tanager (Dubusia taeniata). Species comprise three families and five genera.

Fig. 3

Fig. 3. Comparisons of A) species richness and B) species diversity, represented by the Simpson Index, of mixed species flocks from three habitat types. Different letters indicate significant differences among habitat types according to posthoc pairwise differences estimated by least square means.

Fig. 4

Fig. 4. Canonical correspondence analysis (CCA) ordination showing the species composition of mixed species flocks from three habitat types. The ordination was constrained by habitat type. The variance explained by each CCA axis is included (%). Ellipses represent the standard deviation around the centroids of the factor constraints (habitat types), with the solid, dotted, and dashed lines representing native forest, non-native forest, and native shrubs respectively. Species scores can be found in Table S3.

Fig. 5

Fig. 5. Comparisons of standardized effects sizes (SES) of A) functional richness, B) function diversity, represented by the Rao Index, and C) functional uniqueness of mixed species flocks from three habitat types. Different letters indicate significant differences among habitat types according to posthoc pairwise differences estimated by least square means (Table S4).

Fig. 6

Fig. 6. Comparisons of the community weighted means (CWM) for five functional traits of mixed species flocks from three habitat types. Bars represent the CWM weighted by the abundance of the different species in a flock, and error bars show standard errors. Different letters indicate significant differences among habitat types according to posthoc pairwise differences estimated by least square means (Table S5).

Table 1
Table 1. Descriptions of five functional traits measured from specie participating in mixed species flocks used for functional diversity analyses.
| Functional Trait | Functional Role | Description | Interpretation |
| Body mass | Life history1,2 | Body mass of the species | Proxy for body size; larger values indicate larger birds |
| Bill index | Foraging3 | Bill width by total culmen length | Values >1 indicate wider, shorter bills; values <1 indicate longer, narrower bills |
| Hand-wing index (HWI) | Movement, foraging4 | Difference between length of primaries and secondaries by length of primaries | Proxy for wing length and aspect ratio; larger values indicate greater flight efficiency and dispersal ability |
| Tail index | Movement, foraging5 | Length of longest rectrix by body mass | Measure of tail length relative to body size; larger values indicate longer tails |
| Tarsus index | Movement, habitat usage6 | Tarsus length by body mass | Measure of tarsus length relative to body size; larger values indicate longer legs |
| 1Peters (1983), 2Lewis et al. (2008), 3Friedman et al. (2019), 4Sheard et al. (2020), 5Thomas and Balmford (1995), 6Zeffer et al. (2003) | |||
Table 2
Table 2. The frequency of species’ participation in mixed-species flocks from three habitat types. Shown are the 10 most frequent flock participants listed from most to least frequent in all flocks observed during transect surveys.
| Species | Native Forest Flocks | Regenerating Forest Flocks | Non-native Forest Flocks | All Flocks | |||||||||
| Common and Scientific names | Total number of flocks | Number of individuals per flock (mean ± SD) | Flocking frequency (%) | Total number of flocks | Number of individuals per flock (mean ± SD) | Flocking frequency (%) | Total number of flocks | Number of individuals per flock (mean ± SD) | Flocking frequency (%) | Total number of flocks | Number of individuals per flock (mean ± SD) | Flocking frequency (%) | |
| Spectacled Redstart | 84 | 2.2 ± 0.9 | 66.7 | 95 | 2.4 ± 1.2 | 82.6 | 86 | 2.4 ± 1.3 | 76.1 | 265 | 2.3 ± 1.2 | 74.9 | |
| Myioborus melanocephalus | |||||||||||||
| Superciliaried Hemispingus | 68 | 2.0 ± 1.1 | 54.0 | 54 | 1.6 ± 0.9 | 47.0 | 53 | 1.9 ± 0.9 | 46.9 | 175 | 1.8 ± 1.0 | 49.4 | |
| Thlypopsis superciliaris | |||||||||||||
| Masked Flowerpiercer | 55 | 1.7 ± 0.9 | 43.7 | 43 | 1.5 ± 0.7 | 37.4 | 63 | 1.7 ± 0.8 | 55.8 | 161 | 1.6 ± 0.8 | 45.5 | |
| Diglossa cyanea | |||||||||||||
| Black-crested Warbler | 59 | 1.6 ± 0.6 | 46.8 | 37 | 1.3 ± 0.5 | 32.2 | 39 | 1.3 ± 0.7 | 34.5 | 135 | 1.5 ± 0.7 | 38.1 | |
| Myiothlypis nigrocristata | |||||||||||||
| Black Flowerpiercer | 45 | 1.6 ± 1.0 | 35.7 | 33 | 1.3 ± 0.5 | 28.7 | 46 | 1.6 ± 0.8 | 40.7 | 124 | 1.5 ± 0.8 | 35.0 | |
| iglossa humeralis | |||||||||||||
| Yellow-breasted Brushfinch | 37 | 1.8 ± 1.3 | 29.4 | 46 | 1.8 ± 0.7 | 40.0 | 19 | 1.5 ± 0.6 | 16.8 | 102 | 1.7 ± 1.0 | 28.8 | |
| Atlapetes latinuchus | |||||||||||||
| Scarlet-bellied Mountain Tanager | 50 | 1.4 ± 0.7 | 39.7 | 27 | 1.5 ± 0.5 | 23.5 | 14 | 1.5 ± 0.8 | 12.4 | 91 | 1.5 ± 0.6 | 25.7 | |
| Anisognathus igniventris | |||||||||||||
| Russet-crowned Warbler | 37 | 1.4 ± 0.7 | 29.4 | 18 | 1.2 ± 0.5 | 15.7 | 34 | 1.4 ± 0.9 | 30.1 | 89 | 1.4 ± 0.7 | 25.1 | |
| Myiothlypis coronata | |||||||||||||
| Pearled Treerunner | 31 | 1.8 ± 0.8 | 24.6 | 30 | 1.8 ± 0.8 | 26.1 | 14 | 1.5 ± 0.5 | 12.4 | 75 | 1.8 ± 0.7 | 21.2 | |
| Margarornis squamiger | |||||||||||||