The following is the established format for referencing this article:
Parsons, M. A., S. T. Meiman, S. A. Munoz, and A. Bridges. 2022. Nest survival and cause-specific nest mortality in the San Clemente Bell’s Sparrow. Journal of Field Ornithology 93(4):2.ABSTRACT
Nest monitoring and the use of camera systems can provide researchers with reproductive data integral to the successful management and recovery of threatened and endangered avian species. From 2012 to 2019, we used cameras to monitor 110 nests of the threatened San Clemente Bell’s Sparrow (Artemisiospiza belli clementeae) to identify nest predators and evaluate threats to their recovery on San Clemente Island (SCI). We evaluated the effects of winter precipitation, nest initiation date, and nest substrate on nest survival rates. We also used cameras to identify and estimate cause-specific mortality rates from native and non-native nest predators. We did not observe significant impacts of precipitation or nest initiation date on nest survival. Nests in California boxthorn (Lycium californicum) had significantly lower survival rates than nests in other substrates. The native San Clemente Island fox (Urocyon littoralis clementae) was the most common nest predator, followed by non-native black rats (Rattus rattus). Overall, nests were much more likely to be predated by native than non-native predators. This suggests that San Clemente Bell’s Sparrows could achieve recovery despite the presence of non-native predators. Our findings help describe and quantify the breeding ecology of San Clemente Bell’s Sparrows, providing necessary information for data-driven management and recovery efforts.RESUMEN
INTRODUCTION
Long-term monitoring programs allow for assessment of population dynamics and identification of threats, which ultimately informs adaptive management strategies for species of conservation concern (Brook and Kikkawa 1998, Sinclair et al. 2002). Additionally, for recovering species, monitoring is necessary to track progress toward recovery goals and ensure new threats do not arise undetected (Hoekstra et al. 2002).
Traditional monitoring techniques can be supplemented by newer technologies that improve the feasibility and accuracy of long-term monitoring programs. In avian research, a number of advances have improved researchers’ ability to monitor migration, breeding ecology, nest survival, and causes of mortality (Chiavacci et al. 2018, Imlay et al. 2021, Schreven et al. 2021). Nest cameras have become a valuable tool for providing continuous observations of nest status, limiting the need for researchers to visit the nest. Cameras often allow for definitive identification of the causes of nest failures, even if multiple predators leave physical signs at a nest (McKinnon and Bêty 2009, Liljesthröm et al. 2014, Bridges et al. 2015). These advantages allow for more precise estimation of nest survival rates and cause-specific mortality than were possible using traditional nest monitoring techniques.
We used nest cameras over an eight-year period to monitor nest survival and cause-specific mortality of the San Clemente Bell’s Sparrow (Artemisiospiza belli clementeae). The Bell’s Sparrow (A. belli) ranges throughout California and the Baja California Peninsula, primarily in coastal sage-scrub habitat. Though abundant in some areas (Misenhelter and Rotenberry 2000), Bell’s Sparrows are a species of conservation concern in California (Stephenson and Calcarone 1999), and face a multitude of threats including habitat fragmentation, urbanization, climate change, and invasive species (Akçakaya et al. 2005). The San Clemente Bell’s Sparrow, a non-migratory subspecies endemic to San Clemente Island (SCI; Willey 1990, Turner 2009), is currently listed as Threatened under the Endangered Species Act (U.S. Department of the Interior 1977). The San Clemente Bell’s Sparrow’s decline is primarily attributed to destruction of their habitat by non-native herbivores and predation by non-native predators (U.S. Department of the Interior 1977, Willey 1990, Turner 2009). The United States Navy, which owns San Clemente Island, has funded and overseen systematic population monitoring and recovery efforts of the San Clemente Bell’s Sparrow from 1999 to 2022.
San Clemente Bell’s Sparrows face a multitude of threats both similar to and unique from mainland populations. Annual nest success estimates for San Clemente Bell’s Sparrows from 2009 to 2019 averaged 0.55 (range 0.40–0.75; Meiman et al. 2020), higher than those reported from mainland populations (0.26–0.40; Reynolds 1981, Rotenberry and Wiens 1989, but see Misenhelter and Rotenberry 2000). Compared with mainland Bell’s Sparrows, San Clemente Bell’s Sparrows face a relatively depauperate predator community, consisting of the native San Clemente Island fox (Urocyon littoralis clementae), common raven (Corvus corax), and island night lizard (Xantusia riversiana reticulata; Bridges et al. 2015), as well as non-native feral cats (Felis catus) and black rats (Rattus rattus). Snakes and ground squirrels are the primary predators of mainland Bell’s Sparrow and Sagebrush Sparrow (A. nevadensis) nests (Rotenberry and Wiens 1989, Misenhelter and Rotenberry 2000); both predators are absent from SCI. Native vegetation cover on SCI was largely destroyed by non-native herbivores, which were removed from SCI during the 1970s–1980s and ultimately eradicated by 1991 (Keegan et al. 1994). Since then, recovering vegetation has expanded the breeding range and nesting substrates of Bell’s Sparrows (Meiman et al. 2020).
Climatic and seasonal patterns also likely impact Bell’s Sparrow nest success (Hudgens et al. 2011). The effects of precipitation on avian nest success are complex and variable. In some systems, nest success is positively related to rainfall, possibly related to increased food and water availability or increased nest concealment. However, precipitation can also negatively impact nest success through altered predator communities and habitat characteristics. Precipitation may also have minimal effect on nest success itself, but affect demographics by affecting clutch sizes or number of nesting attempts (Coe and Rotenberry 2003). Drought conditions may threaten Bell’s Sparrows by limiting food availability in dry years (Sullivan et al. 2016) and shortening the nesting season. However, predator populations on the island are also likely related to precipitation, potentially increasing predation risk during wetter periods. Seasonality may also be important, because food availability in desert systems may also decline later in the nesting season as moisture declines, particularly during dry years (Ruth and Skagen 2018).
From 2012 to 2019, we monitored 110 Bell’s Sparrow nests with continuously operating video cameras. We examined the effects of precipitation, nesting substrate, and seasonality on nest success and cause-specific mortality. We expected predation to be the predominant cause of nest failure based on personal experience and evidence from other systems (Rotenberry and Wiens 1989). We hypothesized increased resource availability and increased predator populations following wet winters would reduce nest abandonment but increase nest predation (Sullivan et al. 2016; M. A. Parsons, C. J. Wooden, A. S. Bridges, and D. K. Garcelon, 2019, unpublished manuscript). Although previous research found SCI Bell’s Sparrows nested almost exclusively in California boxthorn (Lycium californicum)–dominated habitat (Kaiser et al. 2009, Hudgens et al. 2011), during this study period the sparrows also occupied the recently recovered alternative shrub cover, including sagebrush (Artemisia californica and A. nesiotica). We expected higher nest success in sagebrush than other substrates, given Bell’s Sparrows affinity for sagebrush in other systems (Rich 1980, Misenhelter and Rotenberry 2000), and observed higher nest success in taller shrubs (Meiman et al. 2020). We expected that early-season nests would have higher survival than late-season nests, given that research in semi-desert species suggests resources may decline late in the breeding season (Ruth and Skagen 2018). Finally, we expected non-native black rats and native San Clemente Island foxes would be the predominant predators based on their spatial overlap with Bell’s Sparrow breeding habitat (M. A. Parsons, personal observation).
METHODS
Study area
SCI, the southernmost of the California Channel Islands, is located approximately 125 km northwest of San Diego, California. Approximately 34 km long, SCI measures 7 km at its widest point and 145 km² in area. The topography of SCI includes a broad plateau along the spine, marine terraces sloping to the western shore, and steep escarpments to the eastern shore. The elevation ranges from 0 to 600 m with the southern portion of SCI dissected by deep canyons. From 2006 to 2019, SCI received an average of 21.5 cm rainfall annually (range 6.1–37.0 cm), the majority of which fell between November and April (Phillips et al. 2007; San Diego State University Soil Ecology and Restoration Group, unpublished data). Mean summer and winter temperatures were 18° C and 14° C, respectively (U.S. Department of the Navy 2013).
During the period of this study, the dominant plant communities included native and non-native grasslands on the upper plateau and maritime desert scrub along western terraces and eastern slopes. The maritime desert scrub habitat at low elevation on the western side consisted of California boxthorn, interspersed with forbs and cactuses, including prickly-pear (Opuntia littoralis and Opuntia oricola), coastal cholla (Cylindropuntia prolifera), and golden-spined cereus (Bergerocactus emoryi; S. T. Meiman, S. A. Munoz, E. E. Deleon, B. Sandstrom, S. Nefas, and A. S. Bridges, 2017, unpublished manuscript). Bell’s Sparrows have historically nested in this boxthorn-associated community at high densities (Meiman et al. 2020). Along the higher elevation western terraces and eastern slopes, various combinations of cactus and forbs occurred, with small patches of low shrubs, the most common of which were sagebrush, coyote brush (Baccharis pilularis), and island morning glory (Calystegia macrostegia amplissima; Meiman et al. 2017, unpublished manuscript). Additional information on the vegetation of SCI can be found in the SCI Integrated Natural Resources Management Plan (U.S. Department of the Navy 2013) and Raven (1963).
Data collection
San Clemente Bell’s Sparrows build nests in low shrubs or other sturdy vegetation, with 39 plant species identified as nesting substrates (Kaiser et al. 2009, Meiman et al. 2020). Females lay one to five eggs, and the average incubation period lasts 12 days. Both parents feed nestlings invertebrates, and fledging occurs approximately 11 days after hatching (Kaiser et al. 2009). The length of the nesting season is positively correlated with precipitation, with nesting seasons lasting up to 177 days (Meiman et al. 2020), allowing the sparrows to successfully nest up to five times during a breeding season (J. T. Stahl, A. S. Docherty, A. S. Bridges, B. R. Hudgens, and D. K. Garcelon, 2010, unpublished manuscript). Bell’s Sparrows are not known to reuse nests, and researchers have documented consecutive nests built in the same shrub as the first nest, or in shrubs up to 100 m away (S. E. Ehlers, L.S. Duval, A. S. Bridges, B. Hudgens, and D. K. Garcelon, 2013, unpublished manuscript).
This study period included two different sampling designs that relied on territory mapping to find Bell’s Sparrows and their nests: the first, in 2012, sampled six plots in high density sparrow areas, with plots ranging from 19 to 34 ha. The second, from 2013 to 2019, randomly sampled at least 104 plots annually from a grid that encompassed the entire island, in which plots ranged from 2 to 23 ha, averaging 12.1 ha. Both of these survey types included nests located by observing pairs of Bell’s Sparrows displaying nesting behavior (e.g., carrying nesting material or food) and then tracking them to their nests.
We selected a subset of nests for video camera nest monitoring. We selected nests for monitoring based on whether the nest area could be consistently and safely accessed by researchers for camera maintenance, and whether the nest substrate provided adequate concealment for the camera without blocking nest access routes for attending adult sparrows. Each video system consisted of a camera (SURE CAM2-IR Miniature Bullet Camera, Nottinghamshire, UK) and digital video recorder (DVR, ARC-19114, ArcVision, Technology Corp., Santa Fe Springs, California) powered by a 12-volt battery. The DVR and battery were located in a weatherproof station located approximately 50 m from the nest and were attached to the nest monitoring camera with 50–150 m of cable. We installed video cameras 10–30 cm from the nest cup, secured the camera to surrounding branches with zip ties, and concealed the cable with vegetation to prevent predators from following camera cables to locate nests. We took different paths to and from the nest to prevent dead-end trails stopping at the nest site. We installed cameras during the incubation phase or within one day of hatching. We completed camera installations in < 10 minutes from the time the attending female flushed from the nest. After installation, we observed the nest from ≥ 50 meters through a video monitor to ensure the female returned to the nest and resumed incubation. If the female did not resume incubation within 45 minutes from the time she initially flushed, we removed the camera (n = 3). In these instances, we checked the nest on the following day to determine if the female was still incubating or brooding. After installing a camera, we visited its DVR and battery station every two days to replace the DVR memory card and battery, and to check the status of the camera and nest. We reviewed video footage to determine the daily nest status (active or inactive) and camera status (functional or non-functional) for each day between camera installation and nest fledging or failure. All personnel installing cameras were qualified under United States Fish and Wildlife Service permit TE-744878-16.1 held by the Institute for Wildlife Studies.
Winter (November–April) precipitation data were collected via five manual rain gauges that were monitored by the San Diego State University Soil Ecology and Restoration Group on San Clemente Island (San Diego State University, unpublished data), and totals for each station were averaged for an island-wide metric. Winter rainfall ranged from 5.73 cm in 2017–2018 to 34.16 cm in 2016–2017.
We categorized nesting substrate as boxthorn, sagebrush, or other nesting substrates. We estimated the beginning (date first egg was laid in a nest) and end (date last known nest fledged or failed) dates for each breeding season and back-calculated the nest initiation date as the start of the incubation period for each nest based on known hatching or fledging dates. Nesting season length is highly variable on SCI (69–177 days during our study). To account for this variability, we scaled nest initiation date by the mean and standard deviation of start days separately within each year.
Data analyses
We initially ran a Bayesian logistic exposure survival model with no covariates to estimate average nest survival excluding covariate effects. We then added covariates to this base model to estimate the overall impacts of precipitation, nest initiation date, and nest substrate. We then ran the multinomial logistic exposure model of Darrah et al. (2018) to estimate cause-specific mortality rates from native predators, non-native predators, and other causes of mortality and relationships between precipitation, nest initiation, and nest substrate to these specific causes of mortality. To account for variation between nesting seasons, we estimated a covariate for each year that was a function of annual precipitation and a random effect for nesting season. We then estimated nest survival as a function of nesting season, linear and quadratic effects of nest initiation date, and nest substrate (boxthorn [n = 75], sagebrush [n = 15], or other [n = 10]). Boxthorn was used as the reference substrate in both models. We used non-informative priors for all parameters: we used a uniform distribution of 0–50 for the random effect variance parameter and normal distributions with a mean of 0 and precision of 0.01 for all fixed effects. We ran three chains of 50,000 iterations, with a burn in of 20,000 and thinned the chains to every third iteration. We evaluated model convergence using Gelman-Rubin diagnostic (Gelman et al. 2014) and by visually inspecting trace plots. We interpreted significance of results based on whether the 90% credible interval of a parameter overlapped 0.
We conducted a post hoc assessment of the relationship between island fox population size and nest predation by island foxes. The size of SCI’s fox population was estimated annually throughout our Bell’s Sparrow nest survival study period (D. A. Green, J. M. Maestas, A. S. Bridges, and D. K. Garcelon, 2019, unpublished manuscript). We compared the proportion of monitored nests that failed due to fox depredation to the island fox population estimate from the previous fall using linear regression to explore whether predation rates may be related to predator population size. We completed all statistical analyses using JAGS 4.3.0 using the jagsUI package (Ver. 1.5.1; Kellner 2019) in program R (Ver. 4.0.4; R Core Team 2021).
RESULTS
We monitored 110 nests with video cameras from 2012 to 2019 (Fig. 1). On average, we deployed 14 (range 6–19) nest monitoring camera systems per year. These cameras monitored each nest for an average of 13 days (range 1–24). Several nests were monitored longer than the expected 23-day nesting period because of asynchronous hatching as well as nestlings staying in the nest longer than the expected 11 days. Of the 110 nests with cameras, 64 (58.2%) successfully fledged at least one young, whereas 46 nests failed (41.8%).
Average daily survival rate throughout the study period was 0.968 ± 0.013 (mean ± standard deviation) for an estimated 23-day survival rate of 0.488 ± 0.117. We did not document a significant effect of precipitation (β = 0.122 ± 0.460) or nest initiation date (β = -0.210 ± 0.212) on nest survival rates. Nests built in boxthorn had lower survival rates than nests built in sagebrush (β = 1.044 ± 0.586) and nests built in other substrates (β = 0.768 ± 0.456; Fig. 2; Table 1).
Video footage allowed us to identify the cause of 41 of 46 camera-monitored nest failures. Of these 41 nests, 35 (85%) were depredated, predominantly by native predators. Among the 35 depredated nests, island foxes were the most common predator (n = 18; 51.4%), followed by black rats (n = 8; 22.8%), island night lizards (n = 4; 11.4%), ravens (n = 4; 11.4%), and deer mice (Peromyscus maniculatus clementis; n = 1; 2.9%). We did not document any nest predations by feral cats, making black rats the only documented non-native predator. Fox and island night lizard predations were distributed across SCI, whereas raven and rat predations took place primarily along the western shore on the north end of SCI. Of the six (15%) nests that failed because of other causes, five nests were abandoned with eggs, and nestlings died in one nest.
The cause-specific mortality model also identified native predators as the primary cause of nest failure. Using boxthorn as the reference substrate, the daily mortality rates for native and invasive predators were 0.023 ± 0.011 and 0.008 ± 0.005 respectively. The daily mortality rate from other, including unknown, causes of mortality was 0.013 ± 0.008. For the full 23-day nesting period, the cause-specific mortality of Bell’s Sparrow nests was 0.394 ± 0.134 and 0.158 ± 0.089 from native predators and non-native predators respectively (Fig. 3; Table 2).
We did not observe a significant relationship between precipitation and any specific cause of mortality. However, predation from non-native predators showed a trend toward increasing with increased precipitation (β = 0.861 ± 0.582). Both nest initiation date and nest substrate showed relationships with specific causes of mortality. Nests built later in the season showed increased predation risk from non-native predators (β = 0.896 ± 0.577). A negative quadratic relationship between nest initiation date and nest failure due to non-predation causes (β = -1.202 ± 0.713), indicated that nest failure due to abandonment or unknown causes peaked in the middle of the nesting season. Predation from invasive predators was lower in sagebrush (β = -8.961 ± 5.811) and other substrates (β = -9.383 ± 5.719) than in boxthorn. These parameters showed wide credible intervals, with all eight predations by non-native predators taking place in nests built in boxthorn. Nest failure from non-predation causes was lower in sagebrush (β = -8.870 ± 5.871) than in boxthorn or other substrates. We did not observe any non-predation failure of nests built in sagebrush (Table 2).
There was no significant relationship between fox population size and proportion of nests depredated by foxes (β = 0.0005, SE = 0.0005, t = 0.857, p = 0.42, r² = 0.109). However, the 2019 nesting season is a notable outlier, and the relationship between fox population size and proportion of nests depredated by foxes is significant if 2019 is excluded (β = 0.0011, SE = 0.0002, t = 5.019, p = 0.004, r² = 0.8344; Fig. 4).
DISCUSSION
San Clemente Bell’s Sparrow daily nests survival rates averaged 0.968 ± 0.013 (mean ± standard deviation) over our study period. This survival rate is average to high relative to other songbird species (Ricklefs 1969, Martin and Pingjun 1992, Jones and Ward 2020) and compared to mainland Artemisiospiza sparrows (Reynolds 1981, Misenhelter and Rotenberry 2000). Our daily survival rate estimate is consistent with previous studies on SCI (Kaiser et al. 2009, Meiman et al. 2020), and this high survival likely reflects the depauperate predator community on San Clemente Island.
Predation by native predators caused the most Bell’s Sparrow nest mortalities, with island foxes the most common predators in all years. Bell’s Sparrows experienced low predation rates by non-native predators, unusual for island bird species (Howald et al. 2007, Bonnaud et al. 2011). Although rats were the second most common nest predator, we did not observe any predation by feral cats, which are common on San Clemente Island (Parsons et al. 2020). Cat and rat control efforts were ongoing on SCI during our study period (L. R. Burlingame, C. J. Wooden, C. Lane, O. Tapia, A. S. Bridges, and D. K. Garcelon, 2018, unpublished manuscript), and these efforts may have reduced non-native depredation impacts. Invasive predator control measures can increase nest success of native birds in both mainland and island systems (Oppel et al. 2014, Weston et al. 2018, Bell et al. 2021). Many island bird species are not adapted to terrestrial predators (Medina and García 2007), but Bell’s Sparrows may be more prepared for these predators because of the presence of island foxes. The two most common mainland predators for Bell’s Sparrows were ground squirrels (Spermophilus spp.) and snakes (Rotenberry and Wiens 1989, Misenhelter and Rotenberry 2000). Neither ground squirrels nor snakes occur on SCI, underscoring the need for biosecurity measures that prevent the introduction of these or other potential Bell’s Sparrow nest predators. The limited predation by non-native predators, along with the relatively high survival rate, suggests that San Clemente Bell’s Sparrows likely have the ability to recover from historical population declines under current conditions.
The relationship between fox depredation rates and fox population size indicates that predator populations may be a factor in nest success, but that other variables are also important. It is unclear to us why the 2019 breeding season experienced such a high rate of nest predation by foxes. One possible hypothesis is high fox reproductive activity following a wet winter, possibly increasing food demand of breeding pairs. Alternatively, it could have been related to the location of monitored nests in 2019, with many nests being monitored along the western shore of the island, which has historically had a high predation rate (Fig. 4).
We also documented an association between nest survival and nest substrate, with nests built in boxthorn having lower survival than nests built in other substrates. This highlights the importance of identifying quality nesting habitat and focusing effort on restoration and maintenance of suitable vegetation cover for Bell’s Sparrows. When feral ungulates were removed from SCI (Keegan et al. 1994), much of the native shrub habitats had been degraded, including sagebrush habitats, although boxthorn remained along the western terraces of SCI (Meiman et al. 2020). We documented lower nest success in boxthorn than other nest substrates, suggesting that continued recovery of sagebrush and other shrubs on SCI could benefit Bell’s Sparrows. Sagebrush in particular may have high value as a nesting substrate because boxthorn is a shorter shrub than sagebrush, which may increase predator encounter rates with Bell’s Sparrow nests in years when resources are fewer, or when shrub cover is sparser (Meiman et al. 2020).
Increased nest predation by non-native black rats on nests built in boxthorn also highlights the value of continued habitat recovery and availability of diverse nesting habitats. As a low shrub, nests built in boxthorn are likely easily accessible to rats. Further, rats are common along the western shore of SCI, where boxthorn is the most common nesting substrate. This spatial overlap further underscores the importance of alternative nesting habitats in different areas of the island.
Kaiser et al. (2009) evaluated the effect of nest substrate on Bell’s Sparrow nest success and found that nests in boxthorn substrates had higher survival than nests in non-boxthorn substrates. However, they sampled exclusively in the boxthorn-dominated west shore habitat; 636 of their 870 nests used California boxthorn as a primary nest substrate, and only a single nest was located in sagebrush. More recent analysis of data collected throughout SCI found no difference between survival of nests placed in boxthorn, non-boxthorn shrubs, and non-shrubs, but did identify a positive relationship between nesting substrate height (sagebrush grows taller than boxthorn) and nest success (Meiman et al. 2020). Our results add additional evidence that sagebrush is a valuable nesting substrate.
Although we did not observe a significant relationship between precipitation and nest success, precipitation may still be an important driver in population dynamics of Bell’s Sparrows. Precipitation can influence a number of factors including primary production, invertebrate availability, predator populations, rodent populations, and nesting season length (Rotenberry and Wiens 1989, Coe and Rotenberry 2003, Dreitz et al. 2012, Conrey et al. 2016). All of these factors may then indirectly affect avian nest success and breeding productivity. These complicated pathways make identifying influences of precipitation on avian demographics extremely challenging. This study emphasized these complicated pathways through differing effects of precipitation on different causes of mortality. No cause was significantly related to precipitation, but non-native predation tended to increase whereas native predation and other causes of mortality tended to decrease with precipitation. This suggests that although overall survival may remain consistent, causes of mortality may vary. On San Clemente Island, precipitation may influence nesting season length in San Clemente Bell’s Sparrows. Extended nesting seasons allow breeding pairs to raise multiple broods and are associated with larger clutch sizes, thus affecting population growth rates. Bell’s Sparrows in particular are known to re-nest multiple times throughout nesting seasons (Stahl et al. 2010, unpublished manuscript). Bell’s Sparrows experience boom and bust population growth patterns (Hudgens et al. 2011) and precipitation contributes to these patterns indirectly through nest season length, predator population dynamics, and food resource availability.
We conducted the first study of cause-specific mortality for the San Clemente Bell’s Sparrow. In contrast with other studies of island birds (Atkinson 1996, Bonnaud et al. 2012), native predators, predominantly foxes, committed most Bell’s Sparrow nest depredations. Despite the presence of both feral cats and black rats, these species were not the most frequent predators of Bell’s Sparrow nests. We also documented a lower nest survival in boxthorn than in other substrates, complementing findings by Kaiser et al. (2009) and Meiman et al. (2020) on Bell’s Sparrow breeding during habitat recovery. This development highlights why continued monitoring of recovering species and habitats is necessary. Continued habitat restoration and recovery is likely the best tool for recovering Bell’s Sparrows on SCI. Although drought is a threat, providing diverse nesting options across the island can buffer against high nest failure in dry years due to variation in precipitation across the island (Meiman et al. 2020). Particularly in dynamic and recovering ecosystems, continued monitoring of recovering species is necessary to successful management. As habitats recover, new habitat relationships of species may be revealed, and as threats are managed, others may emerge (Hudgens et al. 2011). Ecosystems undergoing restoration are often highly dynamic, and it should be expected that patterns may change with properties of the ecosystem.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.ACKNOWLEDGMENTS
We would like to thank the dedicated seasonal and permanent staff of the Institute for Wildlife Studies Bell’s Sparrow Monitoring and Predator Research, Ecology, and Management programs for completing the fieldwork necessary for this manuscript. We are grateful to all the Natural Resource personnel of San Clemente Island for logistical support, input on field methods, and ideas for analyzing this data, particularly N. Desnoyers, J. Stahl, and E. DeLeon. This research was funded by the U.S. Navy Commander, Pacific Fleet. Special thanks to M. Booker for facilitating all aspects of wildlife monitoring and research on SCI and to D. Garcelon for developing nest camera systems and reviewing the manuscript.
DATA AVAILABILITY
The data/code that support the findings of this study are openly available on GitHub at https://github.com/pars2997/BESP-nest-survival.git. All personnel installing cameras were qualified under United States Fish and Wildlife Service permit TE-744878-16.1, held by the Institute for Wildlife Studies.
LITERATURE CITED
Akçakaya, H. R., J. Franklin, A. D. Syphard, and J. R. Stephenson. 2005. Viability of Bell’s Sage Sparrow (Amphispiza belli ssp. belli): altered fire regimes. Ecological Applications 15(2):521-531. https://doi.org/10.1890/03-5378
Atkinson, I. A. E. 1996. Introductions of wildlife as a cause of species extinctions. Wildlife Biology 2(3):135-141. https://doi.org/10.2981/wlb.1996.011
Bell, M. A. N., D. P. Armstrong, J. J. S. Tinnemans, T. E. Rawlence, C. W. Bell, A. McDonald, K. J. Moran, and G. P. Elliott. 2021. The effects of beech masts and 1080 pest control on South Island Robins (Petroica australis). New Zealand Journal of Ecology 45(2):3452. https://doi.org/10.20417/nzjecol.45.53
Bonnaud, E., G. Berger, K. Bourgeois, J. Legrand, and E. Vidal. 2012. Predation by cats could lead to the extinction of the Mediterranean endemic Yelkouan Shearwater Puffinus yelkouan at a major breeding site. Ibis 154(3):566-577. https://doi.org/10.1111/j.1474-919X.2012.01228.x
Bonnaud, E., F. M. Medina, E. Vidal, M. Nogales, B. Tershy, E. Zavaleta, C. J. Donlan, B. Keitt, M. Le Corre, and S. V. Horwath. 2011. The diet of feral cats on islands: a review and a call for more studies. Biological Invasions 13:581-603. https://doi.org/10.1007/s10530-010-9851-3
Bridges, A. S., D. S. Biteman, E. E. Deleon, and B. E. Cross. 2015. Threatened San Clemente Bell’s sparrow nest depredated by island night lizard. Western North American Naturalist 75(2):248-249. https://doi.org/10.3398/064.075.0215
Brook, B. W., and J. Kikkawa. 1998. Examining threats faced by island birds: a population viability analysis on the Capricorn silvereye using long-term data. Journal of Applied Ecology 35(4):491-503. https://doi.org/10.1046/j.1365-2664.1998.3540491.x
Chiavacci, S. J., T. J. Benson, and M. P. Ward. 2018. Linking landscape composition to predator-specific nest predation requires examining multiple landscape scales. Journal of Applied Ecology 55(4):2082-2092. https://doi.org/10.1111/1365-2664.13090
Coe, S. J., and J. T. Rotenberry. 2003. Water availability affects clutch size in a desert sparrow. Ecology 84(12):3240-3249. https://doi.org/10.1890/02-0789
Conrey, R. Y., S. K. Skagen, A. A. Yackel Adams, and A. O. Panjabi. 2016. Extremes of heat, drought and precipitation depress reproductive performance in shortgrass prairie passerines. Ibis 158(3):614-629. https://doi.org/10.1111/ibi.12373
Darrah, A. J., J. B. Cohen, and P. M. Castelli. 2018. A Bayesian multinomial logistic exposure model for estimating probabilities of competing sources of nest failure. Ibis 160(1):23-35. https://doi.org/10.1111/ibi.12510
Dreitz, V. J., R. Y. Conrey, and S. K. Skagen. 2012. Drought and cooler temperatures are associated with higher nest survival in Mountain Plovers. Avian Conservation and Ecology 7(1):6. https://doi.org/10.5751/ACE-00519-070106
Gelman, A., J. B. Carlin, H. S. Stern, D. B. Dunson, A. Vehtari, and D. Rubin. 2014. Bayesian Data Analysis. Chapman and Hall, New York, New York, USA.
Hoekstra, J. M., J. A. Clark, W. F. Fagan, and P. D. Boersma. 2002. A comprehensive review of endangered species act recovery plans. Ecological Applications 12(3):630-640. https://doi.org/10.1890/1051-0761(2002)012[0630:ACROES]2.0.CO;2
Howald, G., C. J. Donlan, J. P. Galván, J. C. Russell, J. Parkes, A. Samaniego, Y. Wang, D. Veitch, P. Genovesi, M. Pascal, A. Saunders, and B. Tershy. 2007. Invasive rodent eradication on islands. Conservation Biology 21(5):1258-1268. https://doi.org/10.1111/j.1523-1739.2007.00755.x
Hudgens, B., F. Beaudry, T. L. George, S. Kaiser, and N. M. Munkwitz. 2011. Shifting threats faced by the San Clemente sage sparrow. Journal of Wildlife Management 75(6):1350-1360. https://doi.org/10.1002/jwmg.165
Imlay, T. L., H. A. R. Mann, and P. D. Taylor. 2021. Autumn migratory timing and pace are driven by breeding season carryover effects. Animal Behaviour 177:207-214. https://doi.org/10.1016/j.anbehav.2021.05.003
Jones, T. M., and M. P. Ward. 2020. Pre- to post-fledging carryover effects and the adaptive significance of variation in wing development for juvenile songbirds. Journal of Animal Ecology 89(10):2235-2245. https://doi.org/10.1111/1365-2656.13285
Kaiser, S. A., E. L. Kershner, and D. K. Garcelon. 2009. Pages 301-313 in The influence of nest substrate and nest site characteristics on the risk of San Clemente sage sparrow nest failure. Proceedings of the 7th California Islands Symposium. Institute for Wildlife Studies, Arcata, California, USA. https://static1.squarespace.com/static/60a6b9c6059cad3139d4d98b/t/615dd765163f2059272e18a7/1633539942334/Kaiser_et_al.pdf
Keegan, D. R., B. E. Coblentz, and C. S. Winchell. 1994. Feral goat eradication on San Clemente Island, California. Wildlife Society Bulletin 22(1):56-61. https://www.jstor.org/stable/3783223
Kellner, K. 2019. jagsUI: a wrapper around “rjags” to streamling “JAGS” analyses. https://cran.r-project.org/web/packages/jagsUI/
Liljesthröm, M., L. Fasola, A. Valenzuela, A. R. Rey, and A. Schiavini. 2014. Nest predators of flightless steamer-ducks (Tachyeres pteneres) and flying steamer-ducks (Tachyeres patachonicus). Waterbirds 37(2):210-214. https://doi.org/10.1675/063.037.0209
Martin, T. E., and L. Pingjun. 1992. Life history traits of open- vs. cavity-nesting birds. Ecology 73(2):579-592. https://doi.org/10.2307/1940764
McKinnon, L., and J. Bêty. 2009. Effects of camera monitoring on survival rates of high-arctic shorebird nests. Journal of Field Ornithology 80(3):280-288. https://doi.org/10.1111/j.1557-9263.2009.00231.x
Medina, F. M., and R. García. 2007. Predation of insects by feral cats (Felis silvestris catus L., 1758) on an oceanic island (La Palma, Canary Island). Journal of Insect Conservation 11:203-207. https://doi.org/10.1007/s10841-006-9036-7
Meiman, S. T., E. E. Deleon, and A. S. Bridges. 2020. Reproductive success of the threatened San Clemente Bell’s Sparrow (Artemisiospiza belli clementeae) in recovering habitats is similar to success in historical habitat. Condor 122:duz071. https://doi.org/10.1093/condor/duz071
Misenhelter, M. D., and J. T. Rotenberry. 2000. Choices and consequences of habitat occupancy and nest site selection in sage sparrows. Ecology 81(10):2892-2901. https://doi.org/10.1890/0012-9658(2000)081[2892:CACOHO]2.0.CO;2
Oppel, S., F. Burns, J. Vickery, K. George, G. Ellick, D. Leo, and J. C. Hillman. 2014. Habitat-specific effectiveness of feral cat control for the conservation of an endemic ground-nesting bird species. Journal of Applied Ecology 51(5):1246-1254. https://doi.org/10.1111/1365-2664.12292
Parsons, M. A., A. S. Bridges, D. S. Biteman, and D. K. Garcelon. 2020. Precipitation and prey abundance influence food habits of an invasive carnivore. Animal Conservation 23(1):60-71. https://doi.org/10.1111/acv.12510
Phillips, R. B., C. S. Winchell, and R. H. Schmidt. 2007. Dietary overlap of an alien and native carnivore on San Clemente Island, California. Journal of Mammalogy 88(1):173-180. https://doi.org/10.1644/06-MAMM-A-015R2.1
Raven, P. H. 1963. A flora of San Clemente Island. Aliso: A Journal of Systematic and Evolutionary Biology 5(3):289-347. https://pdfs.semanticscholar.org/a2fe/0a3c909af1dfa83777a5bf0cc5d0fa69bfcc.pdf
R Core Team. 2021. R: a language for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Reynolds, T. D. 1981. Nesting of the Sage Thrasher, Sage Sparrow, and Brewer’s Sparrow in southeastern Idaho. Condor 83(1):61-64. https://doi.org/10.2307/1367605
Rich, T. 1980. Nest placement in Sage Thrashers, Sage Sparrows, and Brewer’s Sparrows. Wilson Bulletin 92(3):362-368. https://www.jstor.org/stable/4161359
Ricklefs, R. E. 1969. An analysis of nesting mortality in birds. Smithsonian Institute, Washington, D.C., USA. https://doi.org/10.5479/si.00810282.9
Rotenberry, J. T., and J. A. Wiens. 1989. Reproductive biology of shrubsteppe passerine birds: geographical and temporal variation in clutch size, brood size, and fledging success. Condor 91(1):1-14. https://doi.org/10.2307/1368142
Ruth, J. M., and S. K. Skagen. 2018. Reproductive response of Arizona Grasshopper Sparrows to weather patterns and habitat structure. Condor 120(3):596-616. https://doi.org/10.1650/CONDOR-17-128.1
Schreven, K. H. T., C. Stolz, J. Madsen, and B. A. Nolet. 2021. Nesting attempts and success of Arctic-breeding geese can be derived with high precision from accelerometry and GPS-tracking. Animal Biotelemetry 9:25. https://doi.org/10.1186/s40317-021-00249-9
Sinclair, A. R. E., J. M. Gosline, G. Holdsworth, C. J. Krebs, S. Boutin, J. N. M. Smith, R. Boonstra, and M. Dale. 2002. Can the solar cycle and climate synchronize the snowshoe hare cycle in Canada? Evidence from tree rings and ice cores. American Naturalist 141(2):173-198. https://doi.org/10.1086/285468
Stephenson, J. R., and G. M. Calcarone. 1999. Southern California mountains and foothills assessment: habitat and species conservation issues. U.S. Forest Service General Technical Report GTR-PSW-175. U.S. Forest Service, Albany, California, USA. https://doi.org/10.2737/PSW-GTR-172
Sullivan, M. J. P., M. A. Thomsen, and K. B. Suttle. 2016. Grassland responses to increased rainfall depend on the timescale of forcing. Global Change Biology 22(4):1655-1665. https://doi.org/10.1111/gcb.13206
Turner, J. M. 2009. Habitat associations of the San Clemente Sage Sparrow (Amphispiza belli clementeae). Thesis. Humboldt State University, Arcata, California, USA.
U.S. Department of the Interior. 1977. Endangered and threatened wildlife and plants: determination that seven California Channel Island animals and plants are either endangered species or threatened species. Federal Register 42:40682-40685. U.S. Department of the Interior, Washington, D.C., USA.
U.S. Department of the Navy Southwest Division. 2013. Integrated natural resources management plan: Navy Auxiliary Landing Field San Clemente Island, California. San Diego, California, USA. http://tierradata.com/sci/wp-content/uploads/2012/10/SCIINRMP_PublicDraft_INRMP_021113.pdf
Weston, K. A., C. F. J. O’Donnell, P. van dam-Bates, and J. M. Monks. 2018. Control of invasive predators improves breeding success of an endangered alpine passerine. Ibis 160(4):892-899. https://doi.org/10.1111/ibi.12617
Willey, D. W. 1990. Nesting success of San Clemente Sage Sparrows. Southwestern Naturalist 35(1):28-31. https://doi.org/10.2307/3671982
Fig. 1
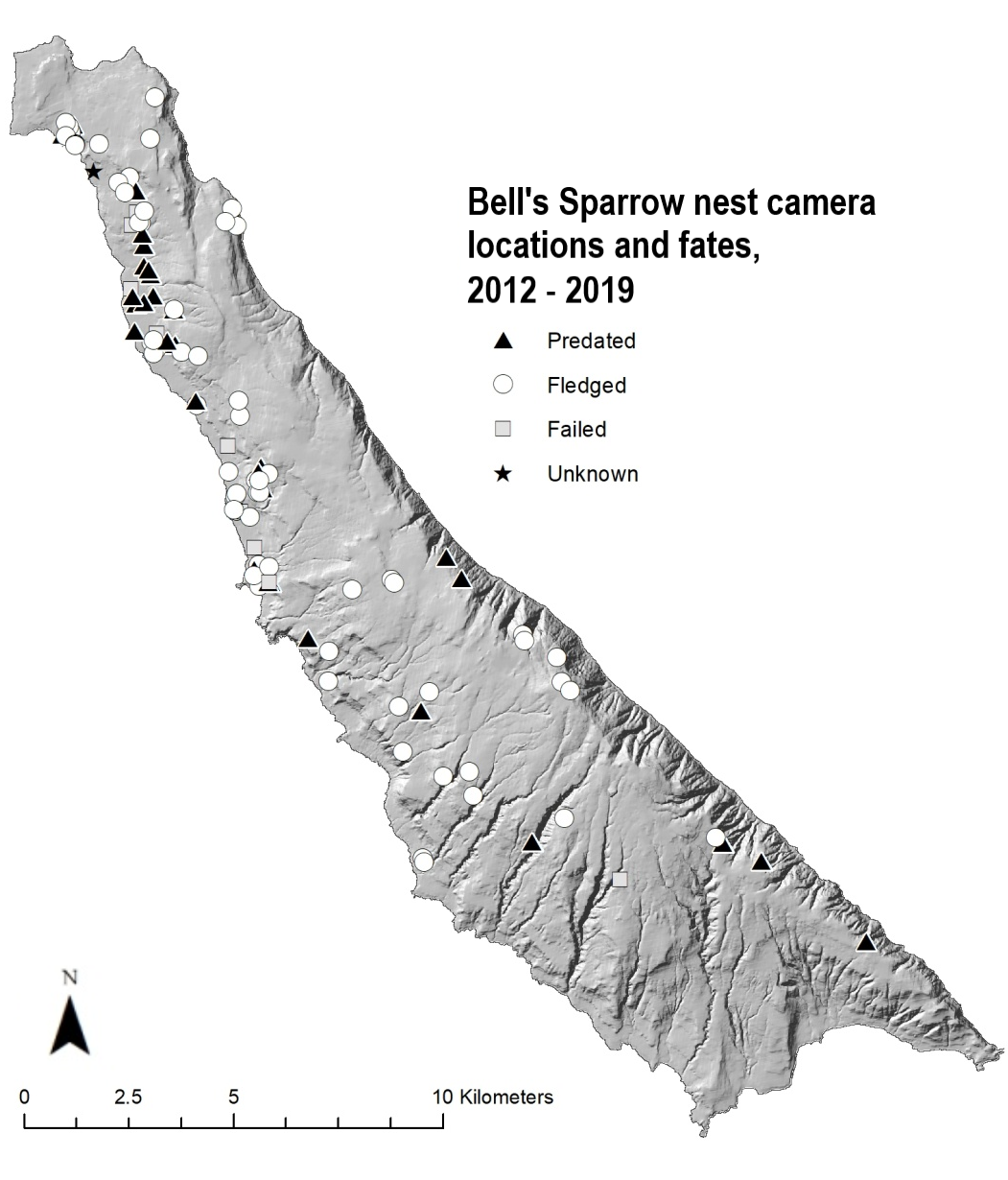
Fig. 1. Nest locations and fates for 110 nests monitored by cameras on San Clemente Island, California, 2012–2019.

Fig. 2
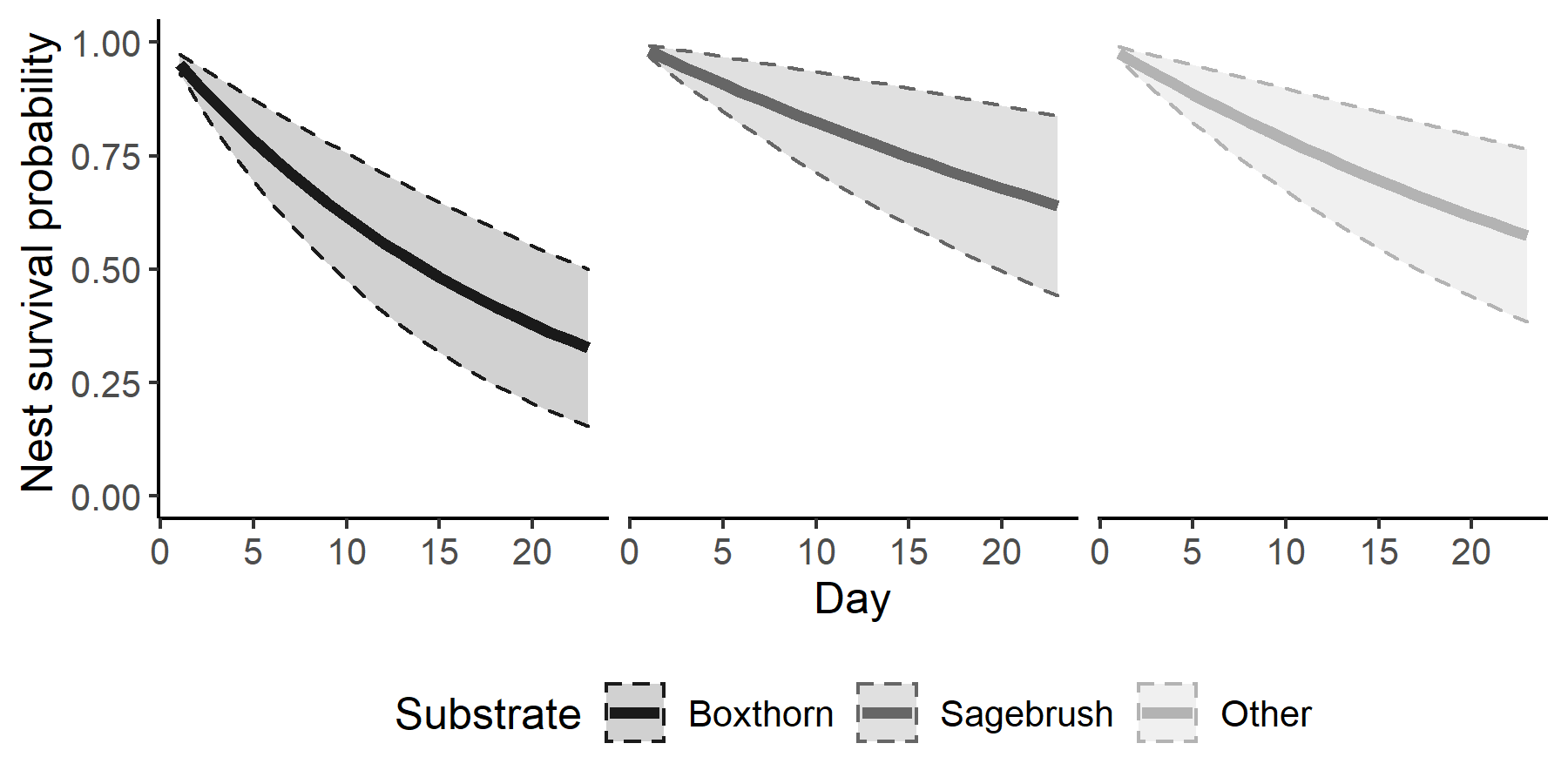
Fig. 2. Nest survival curves for San Clemente Bell’s Sparrow (Artemisiospiza belli clementeae) nests built in boxthorn, sagebrush, and other substrates. Shaded region and dashed lines depict ± 1 standard deviation from the mean estimate. Data are from 110 nests monitored by cameras on San Clemente Island, California, 2012–2019.

Fig. 3
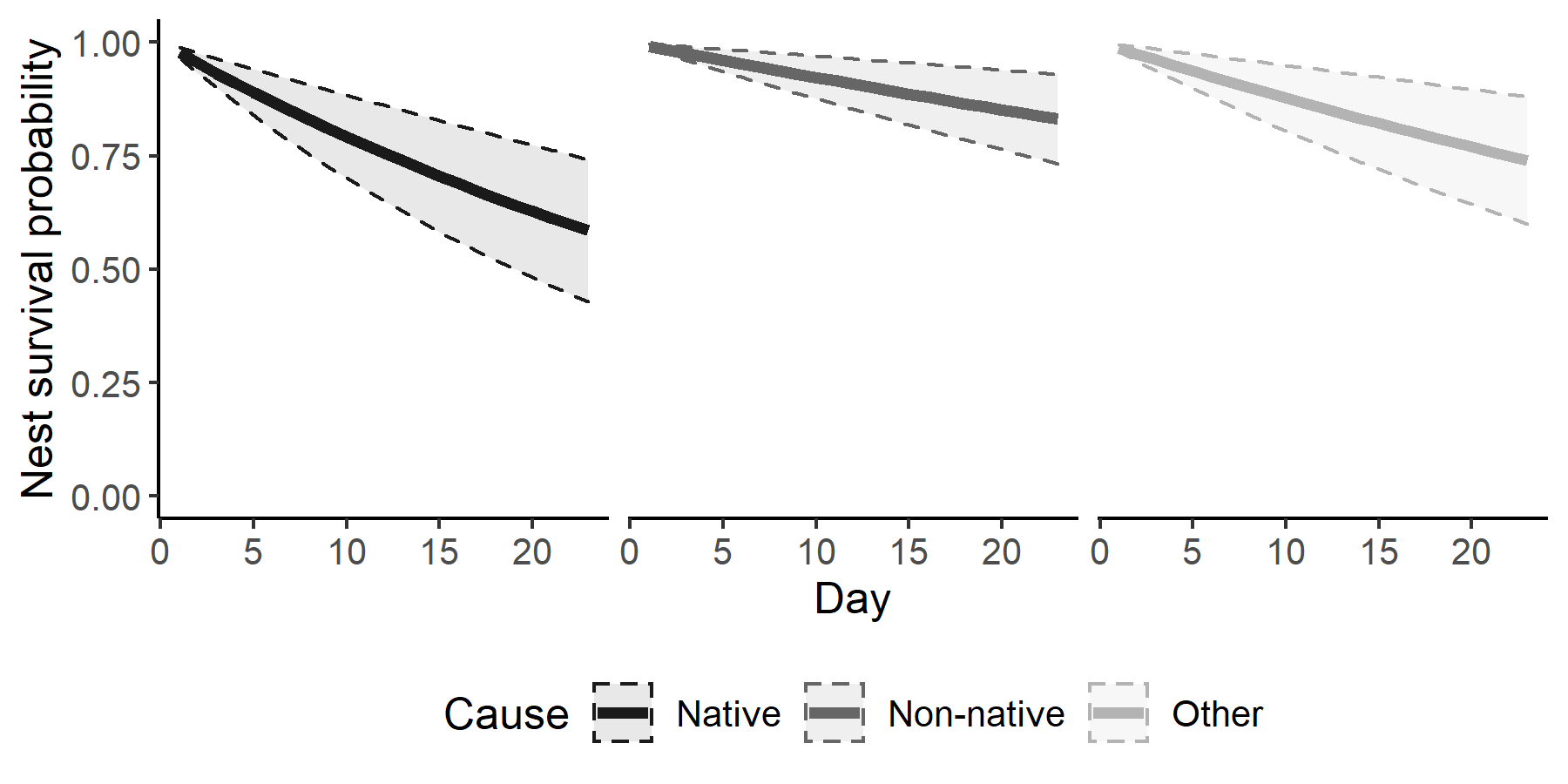
Fig. 3. Nest survival curves for San Clemente Bell’s Sparrow (Artemisiospiza belli clementeae) nests for three causes of mortality: native predators, non-native predators, and other mortalities. Shaded region and dashed lines depict ± 1 standard deviation from the mean estimate. Data are from 110 nests monitored by cameras on San Clemente Island, California, 2012–2019.

Fig. 4
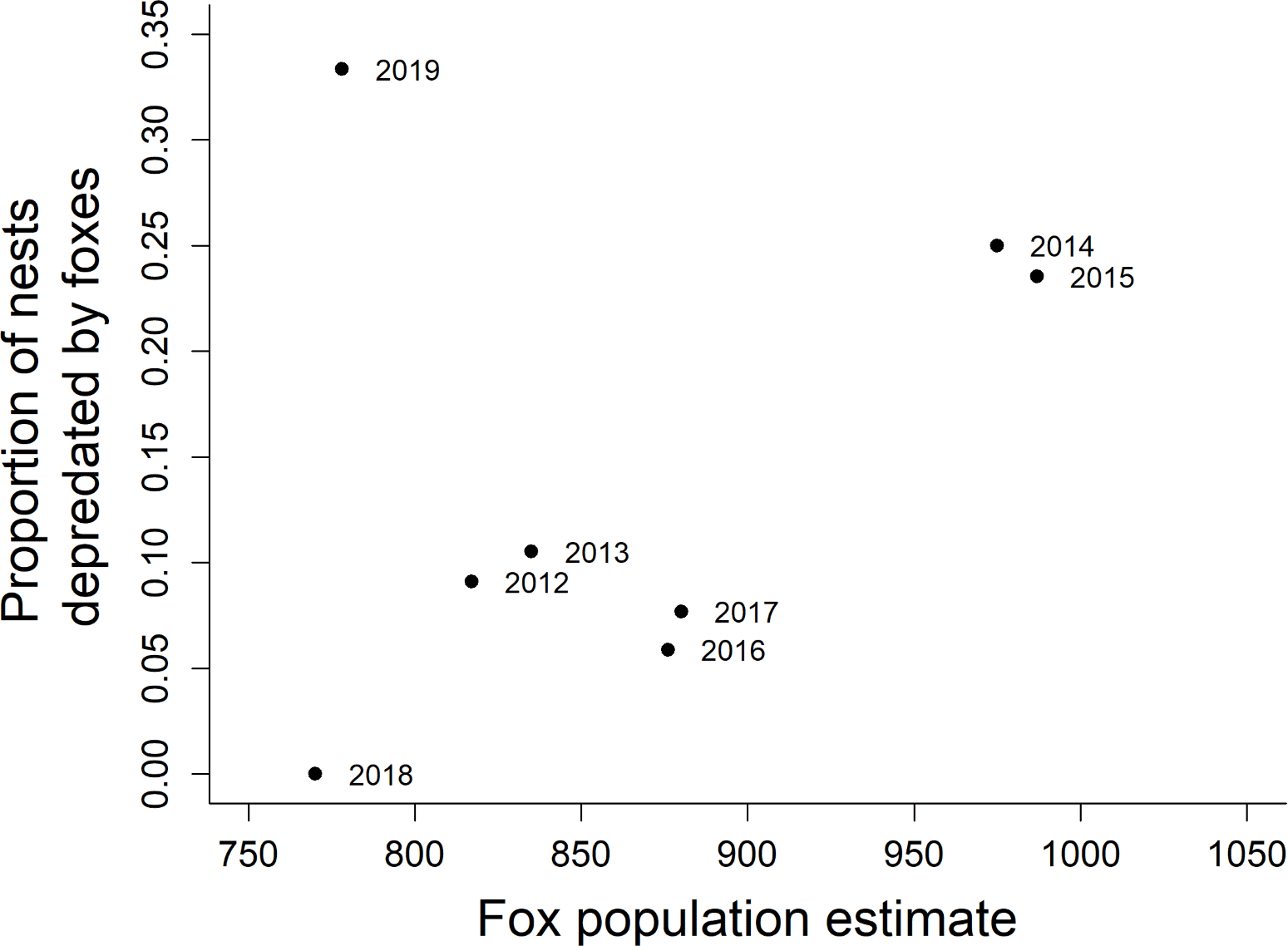
Fig. 4. Proportion of monitored nests depredated by foxes compared to fox population size estimated the fall before each spring nesting season. Data are from 110 Bell’s Sparrow (Artemisiospiza belli clementeae) nests monitored by cameras on San Clemente Island, California, 2012–2019.

Table 1
Table 1. Mean and standard deviation of the posterior distribution and the upper and lower bounds of the 90% credible interval (CI) from the logistic exposure nest survival model. The results are from 110 San Clemente Bell’s Sparrow (Artemisiospiza belli clementeae) nests monitored from San Clemente Island, California, 2012–2019. Covariates with CIs that do not overlap zero are bolded.
| Parameter | Mean | SD | Lower 90% CI | Upper 90% CI |
| Precipitation | 0.104 | 0.458 | -0.632 | 0.833 |
| Nest initiation date | -0.209 | 0.213 | -0.566 | 0.135 |
| Squared nest initiation date | 0.195 | 0.164 | -0.065 | 0.474 |
| Sagebrush | 1.051 | 0.585 | 0.161 | 2.080 |
| Other substrate | 0.773 | 0.462 | 0.044 | 1.563 |
| Daily survival rate | 0.953 | 0.022 | 0.916 | 0.979 |
| 23-day survival | 0.362 | 0.146 | 0.133 | 0.616 |
Table 2
Table 2. Mean and standard deviation of the posterior distribution and the upper and lower bounds of the 90% credible interval (CI) from the multinomial cause-specific mortality model. The results are from 110 San Clemente Bell’s Sparrow (Artemisiospiza belli clementeae) nests monitored on San Clemente Island, California, 2012–2019. Covariates with CIs that do not overlap zero are bolded.
| Parameter | Mean | SD | Lower 90% CI | Upper 90% CI |
| Precipitation, native | -0.231 | 0.462 | -0.978 | 0.489 |
| Precipitation, non-native | 0.861 | 0.582 | -0.076 | 1.801 |
| Precipitation, other | -0.912 | 0.775 | -2.268 | 0.227 |
| Nest initiation date, native | 0.082 | 0.268 | -0.352 | 0.53 |
| Nest initiation date, non-native | 0.896 | 0.577 | 0.068 | 1.94 |
| Nest initiation date, other | -0.554 | 0.758 | -1.915 | 0.556 |
| Square nest initiation date, native | -0.147 | 0.221 | -0.524 | 0.203 |
| Square nest initiation date, non-native | -0.444 | 0.336 | -1.033 | 0.057 |
| Square nest initiation date, other | -1.202 | 0.713 | -2.491 | -0.181 |
| Sage, native | -0.285 | 0.619 | -1.348 | 0.647 |
| Sage, non-native | -8.961 | 5.811 | -20.09 | -1.713 |
| Sage, other | -8.87 | 5.871 | -20.074 | -1.575 |
| Other substrate, native | -0.336 | 0.563 | -1.286 | 0.547 |
| Other substrate, non-native | -9.383 | 5.719 | -20.367 | -2.28 |
| Other substrate, other | -0.613 | 0.886 | -2.179 | 0.714 |
| Daily mortality from native predators | 0.023 | 0.011 | 0.009 | 0.042 |
| Daily mortality from non-native predators | 0.008 | 0.005 | 0.002 | 0.017 |
| Daily mortality from other causes | 0.013 | 0.008 | 0.004 | 0.028 |









