The following is the established format for referencing this article:
Rhodes, E. M., J. Borden and J. McCreadie. 2022. Quantification of physiological aging criteria utilizing window strike data. Journal of Field Ornithology 93(4):12.ABSTRACT
Recent studies have been interested in the effects of age on window strike vulnerability in avian species. To accurately assess age-dependent patterns in avian populations, proper aging criteria should be used to allow for comparisons across studies. Recent window strike studies rely heavily on skull ossification, and we were interested in the accuracy of this method compared to other physiological-based age determinations in passerines and non-passerine landbirds. In this study, we quantitatively analyzed three potential aging criteria that can be used for aging specimens: presence/absence of the bursa of Fabricius, skull ossification, and gonadal maturity. To our knowledge, this is the first quantitative comparison of these criteria. While one study did qualitatively compare the number of agreements versus disagreements between these methods, our study expands on this research by implementing a statistical approach. Skull, bursa, and gonad measurements all were significantly and positively correlated with each other. Nevertheless, we did find disagreement between the methods when further exploring their relationships using Generalized Linear Models. For example, when we compared the number of adult females with immature females to test for window strike vulnerability using a Chi-square test, all three aging criteria produced similar results. Adult females showed a statistically higher rate of window strikes than immature females. However, we still suggest caution using only one criterion to age specimens. In summary, while we did find these three aging characters to be highly correlated, disagreement does exist between these characters.RESUMEN
INTRODUCTION
Bird specimens are an excellent resource to ornithologists; nevertheless, determining the age of specimens can prove difficult if solely relying on plumage characters alone. Numerous studies use bird specimens for research, including window strike studies. Window strikes are the second largest anthropogenic cause of bird deaths in North America (Klem 1989, Loss et al. 2014), and current studies estimate window strikes to cause between 100 million to 1 billion avian deaths in the United States annually (Klem Jr 1990, Loss et al. 2014). Of these studies, a select few have examined the role of age with window strikes and have shown age-dependent patterns, with immature birds experiencing higher mortality rates than adults (Hager and Craig 2014, Loss et al. 2014, Kahle et al. 2016). Understanding how age correlates with window strikes is crucial for conservation managers to understand how it affects avian populations. However, no guidelines on best practices for aging prepared specimens or recently deceased birds exist to our knowledge beyond Pyle (1997), and the aging methods used across previous studies are variable. This inconsistency in aging could impact the interpretation of results and make it difficult to extrapolate patterns from data across studies. In this study, we look at the amount of statistical agreement between three methods of aging specimens: skull ossification, presence/absence of the bursa of Fabricius (henceforth referred to as bursa), and gonadal maturity. While the agreement of these three methods has been qualitatively compared (see Davis 1947), this study is the first to our knowledge to quantitatively analyze these aging characters in passerines and non-passerine landbirds.
Skull ossification (also known as skull pneumatization) is a standard aging method used for avian specimens (Miller 1946, Davis 1947, McNeil and Burton 1972, Stewart 1972, Pyle 1997, 2008). It is common to assign the skull a percent estimate (between 0-100%) of ossified to unossified areas of the skull. Skull ossification describes ontogenesis of a bird’s skull during its first calendar year of life (Pyle 1997). Skulls of recently fledged passerines and non-passerine landbirds consist of only one layer of bone. As the bird progresses into its first year, a second layer begins to develop forming spaces (air pockets) in the skull. These two layers of bone are joined by columns, a process coined skull ossification (Miller 1946, Niethammer 1968, McNeil and Burton 1972, Stewart 1972, Pyle 1997). When using Pyle (1997), all North American birds are said to be Hatching-Year (HY) from hatching to December 31st, and thereafter After-Hatching-Year (AHY) (Pyle 1997). Depending on time of year, the latter category in many cases has fully developed skulls although non-passerine landbirds skulls have been reported to never fully ossify (Pyle 1997, 2008). While ossification has been assessed in numerous songbird species (Chapin 1949, Leberman 1970, Stewart 1972, Hamel and Beacham 1983, Wiley and Piper 1992), it has not been quantitatively assessed in conjunction with the bursa or gonadal maturity in passerines and non-passerine landbirds.
The second method of aging birds that has received limited consideration is the presence/absence of the bursa of Fabricius, a key development organ located above the dorsal wall of the cloaca (Davis 1947, Glick 1956, 1960, 1970; Pyle 2008). The bursa of Fabricius is found primarily in immature birds (Linduska 1943; Davis 1947, Glick 1956, 1983, McNeil and Burton 1972) although in some taxonomic groups, it never regresses and therefore is not a reliable age character in these cases (Taibel 1935, Glick 1960). The bursa is associated with sexual maturity and development (Glick 1956, 1960) and is the location of B-cell, or ‘bursal-derived’ cell, production. Glick (1956, Glick et al. 1956) discovered an inverse, negative relationship between the testes and adrenal glands with the bursa by injecting different breeds of Red Junglefowl (Gallus gallus) and ducks with androgens and corticosteroids. It was observed that in direct response to the injections, the bursa began pre-mature involution (Glick 1956, Glick et al. 1956). The discovery of this was significant because the bursa was previously thought to only regress after a bird reached sexual maturity and was not congruent to gonadal maturity (Jolly 1913, Glick 1956, Glick et al. 1956). Based on the experimental evidence, we would expect that a bird with a bursa would have immature gonads. We set out to test the amount of agreement between the bursa, gonads, and skull ossification.
METHODS
Data collection
We obtained 1,164 curated museum records of 110 different species, consisting of both passerines and non-passerine landbirds, from four institutions (Louisiana State University, University of Florida, Mississippi Museum of Natural History, and the University of Georgia) with window strike mortality listed as their cause of death (Table A1.1). Information collected from each specimen included strike location, date of strike, sex, age assigned, gonadal characters, bursa presence/absence, and percentage of skull ossification. Records spanned all months of the year.
Bursa information was recorded as either present or absent as noted by the data associated with the specimen as well as percent ossification of the skull (0-100%). Since specimen data did not have a distinction between mature and immature gonads, we developed standardized criteria for assigning maturity of female gonads based on the information in the database. We were only interested in the determination of mature versus immature gonads based on the morphology and appearance of the internal gonadal structures. Males are characterized by having gonadal symmetry with two testes whereas females have one ovary typically found on the left side exhibiting asymmetry (Guioli et al. 2014). While the gonads begin forming during development, they do not reach full maturity until sometime after fledging, and age at sexual maturity is variable across species (De Magalhães and Costa 2009, Guioli et al. 2014, Herculano-Houzel 2019). We classified female gonads as mature if the ovary contained at least one ovum or if the oviduct was wide and/or convoluted (as opposed to smooth or straight), indicating a prior breeding event. We did not include male gonads in this analysis due to the inability to discern age in testes.
Statistical analyses
We dummy coded the data to where a 100% ossification of the skull was coded as a mature bird (= 1), whereas incomplete ossification (<100%) was coded as immature (= 0). Similarly, the absence of a bursa (= 1) was considered a mature bird and the presence (= 0) as an immature bird. Lastly, fully developed female gonads were coded as mature (= 1) and gonads not fully developed (= 0) indicated an immature bird. We used the Generalized Linear Model (GLM) function of the lme4 package in R to test the agreement between the three aging criteria (Bates et al. 2014, R Core Team 2022). We also included sex as an explanatory variable in two of the GLM models to test for sex-specific effects on skull ossification and presence/absence of the bursa. We did not look for sex-specific effects in gonads since we did not have that information for males. We were unable to add the processor identification as a random variable as this information was lacking for most of the data. We used all the dummy coded data for the GLM models except we kept the percent ossification as a percentage and analyzed it as a numeric variable in the GLM models.
We used a Pearson Correlation to test for the agreement of age classification between skull ossification, presence/absence of the bursa, and gonadal maturity to calculate Pearson’s Correlation Coefficient (r) for all three variables (bursa-gonad, bursa-skull, skull-gonad) (R Core Team 2022) using the dummy-coded data. To test how well these models performed, we also calculated the coefficient of determinations (R2) for all three comparisons using the Linear Model function in R (R Core Team 2022). Additionally, we conducted Chi-square tests to look at patterns with age in females using all three aging criteria (R Core Team 2022). For these tests, we used an expected ratio of three immatures to one adult used by Klem (1989) which was derived from Lack (1954) and Peterson (1963).
RESULTS
Quantification of aging criteria
Skull, bursa, and gonad measurements all showed statistically significant positive correlations with each other (Table 1) (p-values = < 0.001). These positive values between bursa-skull, bursa-gonad, and skull-gonad correlations indicated agreement on estimates of maturity. The proportion of variance for the dependent variable that is explained by the independent variable (R2) was determined between bursa-skull, bursa-gonad, and skull-gonad. The R2 values from highest to lowest were 1) bursa-gonad at 50%, 2) bursa-skull at 46%, and 3) skull-gonad at 39%. To further explore these relationships, we used Generalized Linear Models (Bates et al. 2014). When we tested for the effect of sex on ossification we found for every 1% increase in ossification, the likelihood of a specimen being identified as a male over female increased by 1.5% (1.07-2.10; 95% C.L.) (p = 0.02). When we tested for the effect of gonads (mature or immature gonads as a proxy for age) on percent ossification, for every 1% increase in ossification, we found that a specimen was 12.3% more likely to be aged as mature over immature using gonads (5.28-31.32; 95% C.L.) (p < 0.001). When we tested for the effect of presence/absence of bursa on gonads, specimens with immature gonads were 38.3% more likely to be assigned to having no bursa over bursa (14.49-118.19; 95% C.L.) (p < 0.001). When we tested for the effect of bursa on percent ossification, we found for every 1% increase in ossification, we found that the specimen was 23.8% more likely to be assigned with no bursa (13.77-43.48; 95% C.L.) (p < 0.001). When we tested for the effect of sex on presence/absence of bursa, specimens with bursas were 2% more likely to be identified as male over female (1.45-2.86; 95% C.L.) (p < 0.001).
Age patterns with females
We used Chi-square tests to look at patterns between immature and adult female birds in our dataset using the same three aging criteria. Our purpose in doing so was to test whether using different aging criteria would impact the overall results. We found a statistically significant difference between immature and adult females in the window strike dataset when using gonads as the aging criteria where adult female strikes are higher in the dataset than immatures (Fig. 1) (p-value < 0.001; Χ2 = 84.933). We found a similar result when using bursa as the aging criteria (Fig. 2) (p-value < 0.001; Χ2 = 52.718) as well as when using ossification (Fig. 3) (p-value < 0.001; Χ2 = 56.728).
DISCUSSION
Aging criteria
In this study, we found that all three aging criteria, bursa, skull, and gonads, were positively correlated with one another (p-values = < 0.001). The relationship between gonads and the bursa performed the best with an R2 of 50% and an r value of 71%. However, when we explored this relationship further, there were a few disagreements where some records had no bursas but immature gonads (n = 7 out of 71 records)(Table A1.2). Davis (1947) classified birds with bursas that had mature gonads, also showing disagreement between these two criteria. Additionally, the dataset included some birds with bursas, fully ossified skulls, and mature gonads. Interestingly, Davis did not report female birds with no bursas and immature gonads. Because Davis (1947) does report the numbers of males and females in the study, more study is needed to determine whether there is a sex-specific difference in the relationship between the gonads and the bursa. Furthermore, Davis (1947) did not report how immature versus mature gonads were determined, and we were unable to differentiate between mature and immature male gonads in our dataset. Future research should focus on whether bursal involution and gonadal maturity are congruous in passerines and non-passerine landbirds for males.
The bursa
The bursa is by far an understudied organ in wild songbirds. Studies have determined development and degeneration timing of the bursa in different breeds of Red Junglefowl (Gallus gallus) (Glick 1956, Glick et al. 1956), but there is limited information available on bursal involution in relation to developmental age in wild songbirds (Pyle 2008). While our study elucidated the relationship between the bursa presence/absence with other characteristics such as skull ossification, gonadal maturity, and sex, much work is needed to fully understand its role in wild songbirds. The bursa-gonad relationship had an R2 value of 50%, meaning the two values are positively correlated, but we found that specimens assigned with immature gonads were 38% more likely to be assigned with no bursa when using a Generalized Linear Model. One possible explanation for this disagreement could be females with fully mature gonads were collected prior to the onset of their first breeding effort and thus would not have a convoluted oviduct, an ovum, or presence of prior breeding events. Additionally, it could be explained by processor error and the inability to locate the bursa. To further investigate this, we were interested in the effect that sex may have on bursa assignment. Specimens with bursas were 2% more likely to be identified as male over female (1.45-2.86; 95% C.L.) (p < 0.001). This could be an indication of sex-specific variation in timing of bursa involution of males versus females.
Using skull ossification
Several published studies rely heavily on skull ossification to classify age; some of these use a combination of criteria while others solely used skull ossification to determine age (Kessel 1951, Klem 1989, Hager and Craig 2014, Kahle et al. 2016, Colling et al. 2022). Our analysis revealed that there appears to be some sex variation in the timing of skull ossification. Thus, we advise caution on using this sole criterion based on our results and suggest using an additional criterion to confirm age. Previous research has revealed that the rate of skull ossification is species-dependent (Kessel 1951, Nero 1951, Grant 1966, Leberman 1970, McNeil and Burton 1972, Stewart 1972, Eaton 2001), but we suggest that other variables (such as environmental stressors) could also affect the rate of pneumatization in individuals. We do recognize that our data included records from throughout the calendar year and that most passerines cannot be reliably aged into their second calendar year (Pyle 1997). Since our aging analyses did include ten individuals classified as non-passerine landbirds, we examined these individually to determine if they would have more disagreements with ossification versus gonads and bursa since it has been reported that sometimes their skulls do not fully ossify (Pyle 1997). Of these, only four had disagreements between the bursa and ossification and only one disagreement between the gonads and the skull, thus, not affecting our results overall. Of note, 8 of the 10 individuals were reported as having 100% ossification. Nevertheless, we did find that all three aging criteria produced the same results overall (Fig. 1-3).
Plumage and molt characters
Curated specimens are an important resource for research but there are some limitations with what data specimens can provide such as age based on plumage and molt. Once a specimen has been prepared, it can be difficult without experience to determine age based on plumage and molt due to the inability to extend the wing out and because of natural color wear over time (Doucet and Hill 2009, Carrillo-Ortiz et al. 2021). Thus, we could not add age based on plumage and molt as an additional variable in our dataset. We suggest it would be helpful for avian specimen preparators to include specific molt and plumage information with the specimen records prior to preparation. Testing the agreement of age using molt and plumage in contrast with the three criteria we analyzed, may provide helpful insight into how to assess age more accurately. One reason this information may be excluded by museum curators could be because historically there was no standard method to record molt and plumage observations of a specimen when prepared. While the methods of Pyle (1997) allow researchers to assign an age, the molt and plumage observations are typically not transcribed on specimen labels. However, with the development of the three-letter plumage and molt system by Wolfe et al. (2012), this system could provide a solution (Wolfe et al. 2012, Pyle et al. 2022). Additionally, perhaps it is worth re-evaluating how we designate mature versus immature birds overall, especially when using terminologies found in Pyle (1997) where AHY is typically synonymous with a mature bird and an HY with an immature bird. Perhaps adding a physiological determinate of age alongside molt and plumage information will provide a more informative way to designate a mature or immature bird without using the cut-off date of December 31st to dictate age.
CONCLUSION
To our knowledge, this study is the first quantitative comparison of the bursa, skull, and gonads as aging criteria in passerines and non-passerine landbirds. We found that all three aging criteria were significantly correlated. Nevertheless, our results revealed some level of disagreement between the three aging criteria. Our study supported the findings of Davis (1947) who also found disagreements between these three aging criteria. Therefore, we recommend using more than one character when aging specimens. Finally, we emphasize the importance of avian specimens having detailed, complete records associated with them in museum collections. We suggest avian preparators and collections include date collected, location collected, cause of death, skull ossification, bursa, gonadal characters, and molt and plumage information whenever possible for future investigations into the accuracy of these aging criteria. While our Chi-square results revealed similar patterns with age and window strikes using all three criteria, using only one of these aging criteria could cause an overestimation or underestimation of a particular age group. Future studies should focus on how using molt and plumages as aging criteria correlates with skull ossification, the bursa, and gonadal maturity.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.ACKNOWLEDGMENTS
We would like to thank all the various institutions who allowed us to visit to collect data and thank those that contributed data to this project. We would also like to especially thank Steven Cardiff, Donna Dittmann, Scott Peyton, Matthew Powers, Amanda Carpenter, Frank Moore, and Ylenia Chiari for providing invaluable input and laboratory assistance. This research was funded by the Alabama Ornithological Society through the Dan C. Holliman grant and the Summer Undergraduate Research Fellowship at the University of South Alabama. Dedicated to Katie McCreadie, Clinton Smoot Major, and Brian Axsmith who all left the University of South Alabama family too soon. Thank you for your friendship and mentorship.
DATA AVAILABILITY
Data and code available upon reasonable request
LITERATURE CITED
Bates D., M. Mächler, B. Bolker, and S. Walker. 2014. Fitting linear mixed-effects models using lme4. ArXiv Prepr ArXiv14065823.
Carrillo-Ortiz J., S. Guallar, J. Martínez-Vargas, and J. Quesada. 2021. A revision of bird skin preparation aimed at improving the scientific value of ornithological collections. Avian Biology Research 14:48-54. https://doi.org/10.1177/1758155920987151
Chapin J.P. 1949. Pneumatization of the skull in birds. Ibis 91:691-691.
Colling O., C. Guglielmo, S. Bonner, and Y. Morbey. 2022. Migratory songbirds and urban window collision mortality: vulnerability depends on species, diel timing of migration, and age class. Avian Conservation and Ecolology 17(1):22. https://doi.org/10.5751/ACE-02107-170122
Davis D.E. 1947. Size of bursa of Fabricius compared with ossification of skull and maturity of gonads. J Wildlife Management 11:244-251. https://doi.org/10.2307/3796282
De Magalhães J.P. and J. Costa. 2009. A database of vertebrate longevity records and their relation to other life-history traits. Journal of Evolutionary Biology 22:1770-1774. https://doi.org/10.1111/j.1420-9101.2009.01783.x
Doucet S.M. and G.E. Hill. 2009. Do museum specimens accurately represent wild birds? A case study of carotenoid, melanin, and structural colours in long-tailed manakins Chiroxiphia linearis. Journal of Avian Biology 40:146-156. https://doi.org/10.1111/j.1600-048X.2009.03763.x
Eaton S.W. 2001. Pneumatization of the skull in the Parulidae. Wilson Bulletin 113:273-278. https://doi.org/10.1676/0043-5643(2001)113[0273:POTSIT]2.0.CO;2
Glick B. 1956. Normal growth of the bursa of Fabricius in chickens. Poultry Science 35:843-851. https://doi.org/10.3382/ps.0350843
Glick B. 1960. Growth of the bursa of Fabricius and its relationship to the adrenal gland in the White Pekin duck, White Leghorn, outbred and inbred New Hampshire. Poultry Science 39:130-139. https://doi.org/10.3382/ps.0390130
Glick B. 1970. The Bursa of Fabricius: A Central Issue. BioScience 20:602-604. https://doi.org/10.2307/1295307
Glick B. 1983. Bursa of Fabricius. Avian Biology 7:443-500. https://doi.org/10.1016/B978-0-12-249407-9.50015-6
Glick B., T.S. Chang, and R.G. Jaap. 1956. The bursa of Fabricius and antibody production. Poultry Science 35:224-225. https://doi.org/10.3382/ps.0350224
Grant P.R. 1966. Retarded or arrested cranial development in a Mexican passerine, Myiopagis viridicata (Vieillot). American Midland Naturalist 142-149. https://doi.org/10.2307/2423486
Guioli S., S. Nandi, D. Zhao, J. Burgess-Shannon, R. Lovell-Badge, and M. Clinton. 2014. Gonadal Asymmetry and Sex Determination in Birds. Sexual Development 8:227-242. https://doi.org/10.1159/000358406
Hager S.B. and M.E. Craig. 2014. Bird-window collisions in the summer breeding season. PeerJ 2:e460. https://doi.org/10.7717/peerj.460
Hamel P.B. and J.L. Beacham. 1983. A laboratory study of cranial pneumatization in Indigo Buntings. Journal of Field Ornithology 54:58-66.
Herculano-Houzel S. 2019. Longevity and sexual maturity vary across species with number of cortical neurons, and humans are no exception. Journal of Comparitive Neurology 527:1689-1705. https://doi.org/10.1002/cne.24564
Jolly J. 1913. L’involution physiologique de la bourse de Fabricius et ses relations avec l’apparition de la maturite sexuelle. CR Society Biology 75:638-648.
Kahle L.Q., M.E. Flannery, and J.P. Dumbacher. 2016. Bird-window collisions at a west-coast urban park museum: analyses of bird biology and window attributes from Golden Gate Park, San Francisco. PLoS One 11. https://doi.org/10.1371/journal.pone.0144600
Kessel B. 1951. Criteria for sexing and aging European starlings (Sturnus vulgaris). Bird-Banding 22:16-23. https://doi.org/10.2307/4510224
Klem Jr D. 1989. Bird: Window Collisions. Wilson Bulletin 101:606-620.
Klem Jr D. 1990. Collisions between birds and windows: mortality and prevention (Colisiones de pájaros con ventanas: mortalidad y prevención). Journal of Field Ornithology 120-128.
Lack D. and F. R. S. 1954. The Natural Regulation of Animal Numbers. Oxford: Clarendon Press. London: Geoffrey Cumberlege, Oxford University Press.
Leberman R.C. 1970. Pattern and timing of skull pneumatization in the Ruby-crowned Kinglet. Bird-Banding 41:121-124. https://doi.org/10.2307/4511646
Linduska J.P. 1943. A gross study of the bursa of Fabricius and cock spurs as age indicators in the ring-necked pheasant. The Auk 60:426-437. https://doi.org/10.2307/4079265
Loss S.R., T. Will, S.S. Loss, and P.P. Marra. 2014. Bird-building collisions in the United States: Estimates of annual mortality and species vulnerability. The Condor 116:8-23. https://doi.org/10.1650/CONDOR-13-090.1
McNeil R. and J. Burton. 1972. Cranial pneumatization patterns and bursa of Fabricius in North American shorebirds. Wilson Bulletin 329-339.
Miller A.H. 1946. A method of determining the age of live passerine birds. Bird-Banding 17:33-35. https://doi.org/10.2307/4509901
Nero R.W. 1951. Pattern and Rate of Cranial “Ossification” in the House Sparrow. Wilson Bulletin 63:84-88.
Niethammer G. 1968. Pneumatization of the cranium as a criterion of age. Ibis 110:106-106. https://doi.org/10.1111/j.1474-919X.1968.tb07987.x
Peterson R.T. 1963. The Birds. Life Nature Library. New York: Time. Inc.
Pyle P. 1997. Identification guide to North American birds: a compendium of information on identifying, ageing, and sexing" near-passerines" and passerines in the hand. Slate Creek Press.
Pyle P. 2008. Identification guide to North American birds. Part II. Anatidae to Alcidae. Point Reyes Station. CA: Slate Creek Press.
Pyle P., M. Gahbauer, E.I. Johnson, T.B. Ryder, and J.D. Wolfe. 2022. Application of a global age-coding system (“WRP”), based on molts and plumages, for use in demographic and other studies of birds. Ornithology 139:ukab063. https://doi.org/10.1093/ornithology/ukab063
R Core Team. 2022. R: A language and environment for statistical computing. R Found Stat Comput Vienna Austria.
Stewart R.M. 1972. The Reliability of Aging Some Fall Migrants by Skull Pneumatization. Bird-Banding 43:9. https://doi.org/10.2307/4511821
Taibel A.M. 1935. Esperimento di bursafabriciectomia in Gallus domesticus. Rivista di Biologia 18:416-430.
Wiley R.H. and W.H. Piper. 1992. Timing of Cranial Pneumatization in White-Throated Sparrows. The Condor 94:336-343. https://doi.org/10.2307/1369206
Wolfe J.D., T.B. Ryder, P. Pyle, and E.I. Johnson. 2012. Using molt and plumage cycles to age tropical: updates and recent advances. Ornitologia Neotropical 23:169-174.
Fig. 1
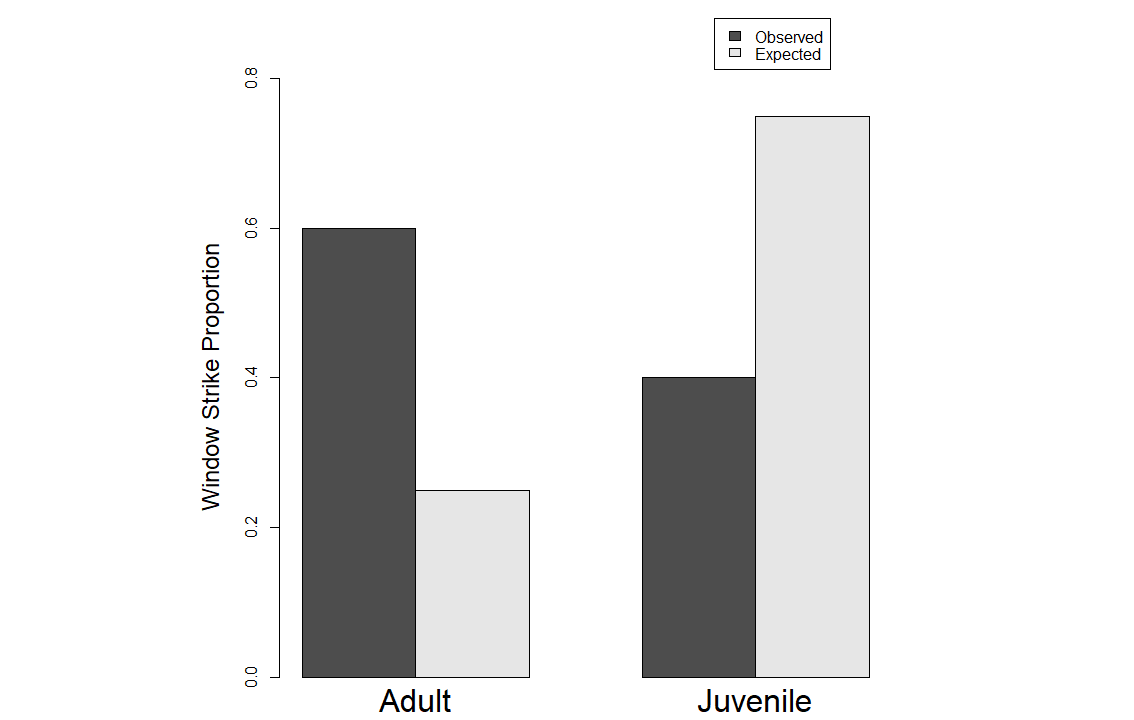
Fig. 1. Results from the Chi-square test using gonads as the determinate of age. Y-axis is the proportion of window strikes and the X-axis is the observed and expected values for both female adults and juveniles.

Fig. 2
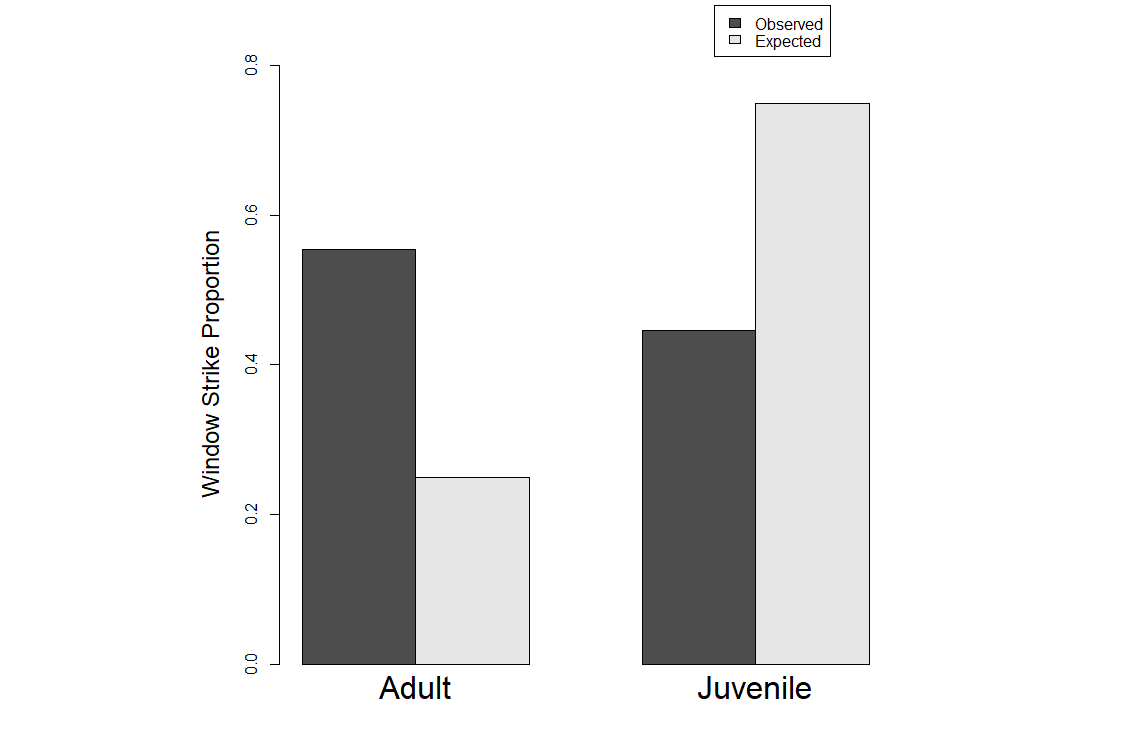
Fig. 2. Results from the Chi-square test using bursa as the determinate of age. Y-axis is the proportion of window strikes and the X-axis is the observed and expected values for both female adults and juveniles.

Fig. 3
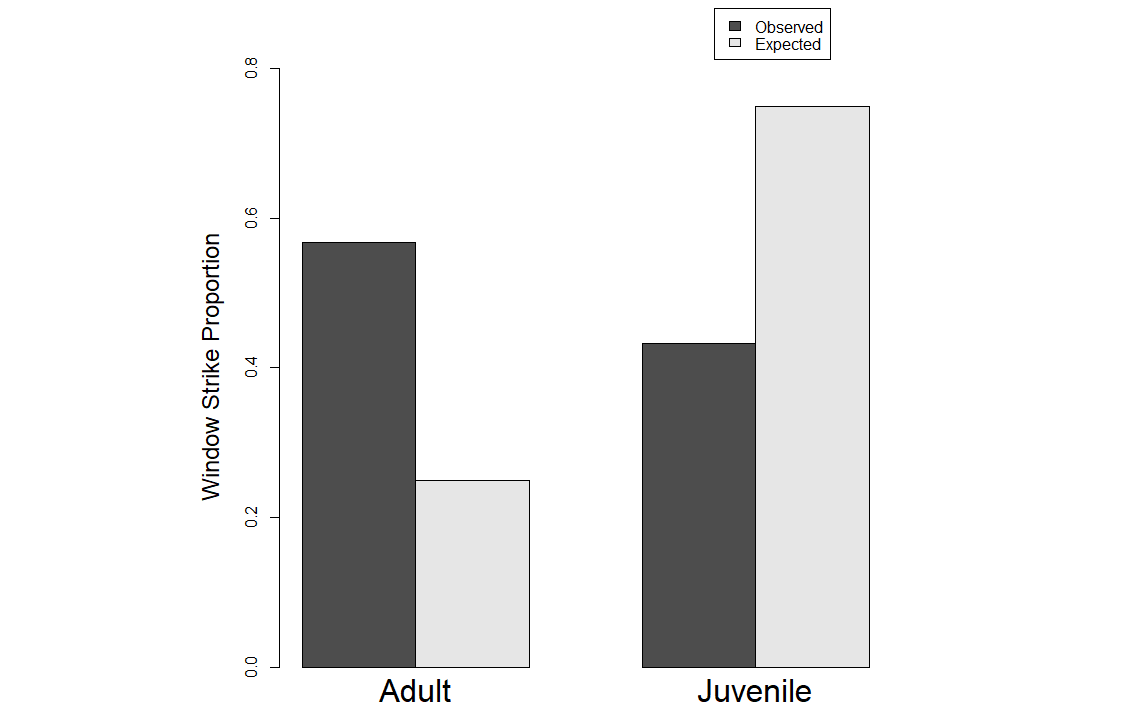
Fig. 3. Results from the Chi-square test using ossification as the determinate of age. Y-axis is the proportion of window strikes and the X-axis is the observed and expected values for both female adults and juveniles.

Table 1
Table 1. Pearson correlations (r), p-values, and Coefficient of determinations (R2) between skull, bursa, and gonadal measures of maturity.
| Pearson correlation (r) | Confidence Interval | Coefficient of determination (R2) | Std. Error | |||||
| Skull | Bursa | Skull | Bursa | Skull | Bursa | Skull | Bursa | |
| Bursa | 0.681 (<0.001) |
(0.631 - 0.725) | 0.464 | (0.043) | ||||
Gonads |
0.625 (<0.001) |
0.706 (< 0.001) |
(0.507 - 0.720) |
(0.608 -0 .783) |
0.390 |
0.499 |
(0.085) |
(0.064) |









