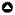The following is the established format for referencing this article:
Brunner, A. R., B. C. Dossman, V. Jirinec, K. L. Percy, C. M. Tonra, E. I. Johnson, and P. P. Marra. 2022. Migratory behavior and connectivity revealed in a secretive Neotropical migratory songbird, the Swainson’s Warbler. Journal of Field Ornithology 93(3):5.ABSTRACT
Improving our understanding of migratory behaviors and connectivity is fundamental for identifying limiting factors and drivers of population decline. With advances in miniaturized tracking technology, we are now able to study these critical aspects of avian ecology, which, for secretive species, was once an exceptional challenge. Here, we identify several unknown aspects of the migratory behavior and connectivity of the elusive Swainson’s Warbler (Limnothlypis swainsonii), by tracking individuals from populations breeding in Louisiana, U.S., and wintering in Jamaica. We identified a migratory divide between the western and eastern portions of the species’ distribution, showing that most Louisiana breeders overwintered in southern Mexico and the Yucatan peninsula, whereas the Jamaica individuals migrated to the eastern portion of the Swainson’s Warbler breeding range. Geolocator data documented that a portion of migratory flights in both populations were sustained well into the day, suggesting that birds migrated over large bodies of water (i.e., Gulf of Mexico and Caribbean Sea) during both spring and fall migration. Furthermore, the phenology and rate of migration differed between populations. Additional research efforts across a broader portion of the range are necessary to better understand the demographic consequences of high migratory connectivity and the implications for the conservation of the species.RESUMEN
INTRODUCTION
The ability to study vulnerable species across the annual cycle (Marra et al. 2015, Culp et al. 2017) is critical to our understanding of the causes of widespread declines of North American bird populations (Rosenberg et al. 2019) at the species level. In recent years, studies looking to determine what factors limit populations of declining migratory species have prioritized both migratory movements (Hewson et al. 2016, Dokter et al. 2018) and the degree to which breeding and wintering populations remain discrete (i.e., migratory connectivity; Webster et al. 2002, Marra et al. 2006). Migration is the most hazardous period of the annual cycle (Sillett and Holmes 2002, Rushing et al. 2017), yet migratory behaviors such as timing and routes are not well described for most migratory songbirds (Faaborg et al. 2010, Marra et al. 2015) despite ongoing advances in tracking technology (McKinnon and Love 2018) and community-science monitoring efforts (Rosenberg et al. 2019). Further, understanding whether a migratory species has connectivity that is strong, e.g.,individuals from a single breeding population migrate to the same nonbreeding location, or weak, e.g., individuals from a single breeding population migrate to several different overwintering locations across the range (Webster et al. 2002, Marra et al. 2006), helps to identify critical periods during the annual cycle, and enables informed decisions that effectively prioritize conservation efforts (Fuller et al. 1998, Sheehy et al. 2010, Marra et al. 2011).
For highly secretive species, collecting even basic ecological data during a single season can be a challenge, and observing migratory behavior and patterns over multiple seasons is all but impossible. Historically, accessing and observing elusive individuals was often too demanding, leading to these species being generally overlooked in field research (Willson and Winne 2016). The recent miniaturization of tracking technology has allowed for the study of secretive migratory species without the need to observe behaviors directly (McKinnon and Love 2018). Additionally, this novel technology enables the study of other aspects of migratory behaviors of small songbirds, such as timing, routes, and connectivity. It is therefore increasingly possible to study the questions critical to the conservation efforts of secretive species, of which many aspects of their full annual cycles are poorly understood.
The Swainson’s Warbler (Limnothlypis swainsonii) is one of the most rare and difficult birds to observe in North America because of its drab plumage, use of dense habitat types, and overall skulky nature (Anich et al. 2020). Swainson’s Warblers are currently listed as a species of conservation concern with population estimates at 140,000 individuals across their range (U.S. Fish and Wildlife Service 2008, Rosenberg et al. 2016). These low numbers are mostly attributed to specific habitat associations, substantial loss and fragmentation of bottomland hardwoods and native bamboo forests across their breeding range, and deforestation of tropical dry forests throughout their winter range (Askins 2000, Twedt and Loesch 2001). Overall, Swainson’s Warbler nonbreeding range is not well described. However, the core winter range consists of islands in the Caribbean Sea and the Yucatan Peninsula (Anich et al. 2020), which is small relative to most other Neotropical migratory warblers. Further, according to the North American Breeding Bird Survey (Sauer et al. 2017), high spatial variability in population trends exist within Swainson’s Warblers’ breeding range. Broadly, notable declines have been observed in patches of the western and northeast portion of their range, whereas probable increases in numbers are seen in the central portion of their breeding range. This restricted range, in addition to habitat loss and low, regionally declining population numbers lends urgency to uncovering more about their population connectivity, full annual cycle ecology, and migratory behaviors.
Here, we equipped both breeding and overwintering Swainson’s Warblers with tracking devices to examine migratory connectivity and the overall timing and probable routes of fall and spring migration, aspects yet unstudied for the species (Anich et al. 2020). To date, much of the information describing the migratory timing and routes (Meanley 1971) are from rare mist net captures during migration (Simons et al. 2004), carcass recoveries (Taylor and Anderson 1973, Crawford 1980, Taylor and Kershner 1986), individual observations during migration (Stevenson and Anderson 1994), and eBird (Sullivan et al. 2009). Given the conservation status of Swainson’s Warblers and the impact different patterns of migration (Hewson et al. 2016) and migratory connectivity (Goldstein et al. 1999, Kramer et al. 2018) have on population trends, our objectives were to (1) determine if populations breeding in Louisiana and overwintering in Jamaica are discrete from one another (i.e., indirect evidence of strong migratory connectivity); (2) identify possible distinctions in the timing of fall and spring departure and migration between the two populations; and (3) observe any differences in migratory routes that might ultimately influence annual survival.
METHODS
Study sites
We conducted this work during both the 2018 and 2019 Swainson’s Warbler wintering season (February to March) in Jamaica and the breeding season (March to July) in Louisiana, U.S. In Jamaica, research was conducted at Font Hill Nature Preserve on the southwest coast (18°02′ N, 77°55′ W) in primarily second growth dry-scrub and scrub-mangrove ecotone habitats. These dry-scrub habitats are dominated by logwood (Haematoxylum campechianum) with areas of dense understory vegetation and tall canopy. Here, ecotone refers to a transitional area between black-mangrove swamp (Avicennia germinans) and scrub, characterized by an open understory and a canopy consisting primarily of logwood and white mangrove (Laguncularia racemosa). In Louisiana, field work during the breeding season was conducted at three sites: Acadiana Park Nature Station in Lafayette (30°15′ N, 91°59′ W), Palmetto Island State Park in Abbeville (29°51′ N, 92°08′ W), and Frenchtown Road Conservation Area in Central (30°28′ N, 90°58′ W). All three sites consisted primarily of mature bottomland hardwood forest with patches of vine tangles at old treefalls and similar, yet slightly varied, understory composition: Acadiana Park has the most open midstory, with scattered patches of palmetto; Palmetto Island has a dense palmetto understory with pockets of cypress swamp; Frenchtown Road has pockets of seasonally flooded cypress-tupelo slough.
Geolocator deployment and retrieval
We captured 20 Swainson’s Warblers (10 each in the breeding and wintering seasons) in 2018 (tag deployment) and 2019 (tag retrieval), in mist nets using conspecific playback of male songs and calls. We banded all birds with a United States Geological Survey (USGS) aluminum band and fitted each breeding individual with a unique color band combination for resighting. Each individual was equipped with an Intigeo stalked light-level geolocator (Model: P50Z11-7-DIP-NOT, Migrate Technology, Coton, Cambridge, UK). Geolocators weighed < 5% of the body mass and were attached with an elastic leg harness (Rappole and Tipton 1991), a standard technique with little evidence for detrimental impacts on birds (Brlík et al. 2019). In the subsequent season (2019 wintering and breeding), we recaptured eight returning individuals (five in Louisiana, three in Jamaica) for data recovery. We used the light data from all eight retrieved geolocators in our analyses. Given that Swainson’s Warblers occupy very large nonbreeding home ranges (Brunner et al. 2022), and that the previous recapture rate for the overwintering population is approximately 70% (2016–2018), it is likely that other individuals with geolocators returned to Jamaica, but we were unable to recapture them.
Nanotag deployment and automated telemetry
During March to April 2016, 2017, and 2019, we tagged 17 individuals overwintering in Jamaica (two in 2016, 10 in 2017, and five in 2019), with 0.43 g (< 5% bird’s body mass) NTQB2-5-1 nanotag radio transmitters (Lotek Wireless, Newmarket, Ontario, Canada) with a modified leg-loop harness using 0.7-mm nylon thread. We passively measured spring departure timing with an array of five automated radio towers across our 2-km plot at Font Hill Nature Preserve in Jamaica. These local receiver stations each consisted of a SensorGnome receiver (https://www.sensorgnome.org) and four horizontally polarized omnidirectional antennas (one receiver was affixed with four 3-element directional Yagi antennas) positioned 9 m high on a galvanized steel mast. Spring migration timing was quantified using detections at our second automated telemetry array located in northern Florida. Each of the six towers in this Florida array consisted of SensorGnome receivers, outfitted with at least two 9-element directional Yagis (PLC 1669, Laird Technologies) facing east and west. The detection range of each tower was approximately 15 km, but up to 25 km under ideal conditions with a clear line of sight between the radio tag and antennas (Mitchell et al. 2012, Taylor et al. 2017). By positioning these stations (approximately 30 km apart) across the narrowest point in Florida, in addition to relying on the broader Motus Wildlife Tracking System in the southeastern U.S. (https://www.motus.org; Taylor et al. 2017), we covered most of the migratory corridor likely used by our tagged birds departing from Jamaica.
Geolocation analysis
We analyzed our light-level geolocator data using methods outlined in Lisovski et al. (2020) and the R package GeolocTools. We downloaded the light data and manually annotated twilights using a threshold adjusted for each individual to conservatively mark outliers for exclusion in final analyses. We used the GeoLight package (Lisovski et al. 2020) to estimate the movement tracks and stationary distributions. Calibrations were made with light-level data collected on tagged birds for 10 to 46 days prior to departure, which, for most birds, occurred before 31 March on the wintering grounds and before 1 August on the breeding grounds (Fig. A1.2, Appendix 1) for individual twilight-error estimates and zenith angles. We set the spatial extent for our models to 70–120 degrees longitudinally and 10–45 degrees latitudinally (Fig. A1.1, Appendix 1). This extent conservatively captured the breeding, wintering, and migratory distributions of Swainson’s Warblers and improved computation efficiency for the particle filter. To determine the timing of both fall and spring migration for most of the individuals equipped with geolocators, we used uninterrupted high intensity light data: full light pattern (FLP) as described in Adamík et al. (2016; Fig. 1). This pattern in the light data can be explained by individuals being in completely open environments and most likely represents diurnal over-water flights of the Louisiana birds crossing the Gulf of Mexico and the Jamaica individuals crossing the Caribbean. Total flight duration for each diurnal movement was estimated by adding the time spent flying during the preceding night (assuming an individual began flying at astronomical twilight) to the total amount of time spent aloft during daylight hours. At times, birds were aloft throughout the entire daylight period: in this case, we estimated the total flight duration to be ~24 hours, which represents a minimum flight bout because birds could have continued to fly through the night.
Estimating migratory behavior, routes, and timing
We uploaded data collected from our automated towers to the Motus Wildlife Tracking System network for preliminary processing, archiving, and dissemination (Taylor et al. 2017). We used the R packages Motus (Brzustowski and LePage 2021) and tidyverse (Wickham et al. 2019) to download, filter, and analyze the data. Time of departure was determined by visually inspecting the departure signals (dB overtime prior to departure) for peak signal strength before its rapid decline and eventual loss sensu (Dossman et al. 2016). The same approach was used to quantify the crossing time of individuals passing Motus towers in Florida or Georgia. With the precise time of departure from Jamaica and with at least one detection in Florida/Georgia, we were able to quantify the minimum amount of time (in days) it took an individual to migrate. We divided that time by the distance (km) between the centroid of our study site and the receiver’s location during migration to estimate a minimum rate of migration in kilometers per day.
RESULTS
Light-level geolocators
Four of the five individuals recaptured in Louisiana overwintered in Central America, with the centroids spanning from the northern Yucatan Peninsula to southern Guatemala (Table 1, Fig. 2). One individual’s wintering distribution overlapped the Yucatan peninsula and western tip of Cuba making it likely to have wintered in either location. The three individuals that were recaptured during the wintering season in Jamaica bred in three distinct locations in the eastern portion of Swainson’s Warbler breeding range (Table 1, Fig. 2). One individual’s centroid was located in North Carolina, one was in eastern Alabama, and the third was centered in north Florida/southeast Georgia (Fig. 2).
By using the FLP data we estimated the timing of both fall and spring migration for six of the individuals equipped with geolocators (Table 1). In the fall, these migratory movements spanned 14 September to 11 October. However, the Jamaica individuals migrated (16 September ± 3.2 days SE) approximately three weeks earlier (18.2 ± 6.4 days SE) than the Louisiana individuals (4 October ± 12.2 days; Table 1). Interestingly, three of the five Louisiana birds started a Gulf of Mexico crossing on the night of 11 October, continuing into the day of 12 October. In the spring, all Louisiana birds, but only one of three Jamaica birds, exhibited these diurnal overwater migratory movements (Table 1). The average timing of these movements for Louisiana birds occurred on 1 April ± 7.5 days (SE) and more than a week later for the one Jamaica individual (10 April). When we calculated total flight duration, starting from departure the night prior into the diurnal flights the following day, Jamaica individuals flew a total of 21.86 hours in the fall (range: 19.25–24.58 hrs; 9.94 daylight hrs) and 14.58 hours in the spring (range: N/A; 3.08 daylight hrs). Louisiana birds flew, on average, 23.72 total hours in the fall (range: 21.66–24.58 hrs; 11.8 daylight hrs) and 19.2 hours in the spring (range: 13.33–24.08 hrs; 7.7 daylight hrs).
Automated telemetry
The timing of spring departure from Jamaica ranged from 31 March to 1 May (average = 13 April ± 9.8 days SE; Table 2) and exhibited a bimodal distribution (Fig. 3B). Twelve of the seventeen birds were detected during migration in Florida and throughout southern Georgia and coastal North Carolina (Fig. 3A). Three of these individuals were detected along the Gulf Coast of Florida, whereas the remaining nine individuals were detected along the Atlantic Coast of Florida. Birds with a northwesterly trajectory on the Gulf Coast migrated more than two weeks earlier (10 April ± 13.4 days SE) than individuals migrating on a northeasterly trajectory (25 April ± 7.1 days SE) and detected on the Atlantic Coast (3A, Table 2). On average, Swainson’s Warblers migrated at a pace of 205.6 ± 107.7 km per day (SE; Fig. 3C) and were detected in Florida during migration from 3 April to 15 May (21 April ± 10.3 days SE; Table 2).
DISCUSSION
With the growing concern over declining migratory bird populations, linking breeding and wintering areas is a critical prerequisite of developing an effective conservation strategy, especially for elusive and understudied species (Rosenberg et al. 2016). The rapid progression in miniaturization of tracking technology (Kays et al. 2015) has made it possible to follow such species across their full annual cycle. We provide a first look into the migratory behaviors and connectivity of individual Swainson’s Warblers from two geographically distinct study populations. By tracking two populations, one of birds breeding in Louisiana and another overwintering in Jamaica, we provide evidence of a longitudinal divide between the western and eastern portions of the species’ distribution. Most Louisiana breeders overwintered in southern Mexico/Yucatan peninsula, whereas the Jamaica individuals were concentrated in the eastern portion of the Swainson’s Warbler breeding range, with the farthest west breeding in Alabama. Additionally, we observed the Louisiana birds making diurnal (i.e., likely over-water) migratory flights about three weeks later in the fall and one week earlier in the spring than their counterparts in Jamaica. Similarly, automated telemetry data from birds wintering in Jamaica suggested that birds migrating in a more northwesterly trajectory migrated a couple weeks earlier than those migrating in a more northeasterly trajectory. Collectively, our tracking data suggests an east-west gradient in the timing of migratory movements across the range of Swainson’s Warblers. Of additional note, geolocator data suggested that both Louisiana and Jamaica individuals regularly take long, direct routes across water (e.g., the Gulf of Mexico and Caribbean Sea) during both spring and fall migration, which has implications for the need of key pre-departure/refueling areas to successfully complete these flights.
The longitudinal separation of populations we observed with Swainson’s Warblers is typical of many migratory passerine species in which strong migratory connectivity has been identified, e.g., Snow Bunting (Plectrophenax nivalis; MacDonald et al. 2012), Common Nightingale (Luscinia megarhynchos; Hahn et al. 2013), Ovenbird (Seiurus aurocapilla; Hallworth et al. 2015), and supports previous research on Swainson’s Warblers that highlighted moderate genetic differentiation between the eastern and western portion of their breeding range (Winker and Graves 2008). Individuals breeding in Louisiana almost entirely winter in the Yucatan and Central America, whereas individuals overwintering in Jamaica were split across the central and east portions of the breeding range. This east-west pattern of connectivity likely indicates that birds overwintering further east of Jamaica (e.g., Bahamas and Puerto Rico) likely breed predominantly in the eastern U.S. (Virginia, North Carolina, etc). Given the spatial variability in population trends (BBS Trend Map, 1966–2015; Sauer et al. 2017), it is crucial to understand how this potential migratory divide and general pattern of connectivity reflect differences in population trajectories on the breeding grounds. This is likely to be an important step to take in future population analyses and becomes especially important if these declines result in the loss of genetic diversity (Winker and Graves 2008).
Two of the five Louisiana birds were found to winter at locations slightly west of their currently known wintering distribution. Although geolocator estimates provide coarse approximations of the wintering locations of these birds, it is important to note that longitudinal estimates tend to be more precise than latitudinal estimates (Lisovski et al. 2020). Further, recent mist netting efforts in Tabasco, Mexico, slightly west of the known wintering distribution, found that Swainson’s Warblers were relatively common in native forests (Winker and Graves 2008, Oliveira et al. 2021). This suggests that our geolocator estimates likely reflect true winter sites and provide more evidence that the wintering range reaches further west than currently described. Despite the rise of community science-based monitoring approaches, even recent updates in their eBird winter range maps (https://ebird.org/science/status-and-trends/swawar/range-map;; Sullivan et al. 2009) fail to accurately capture Swainson’s Warbler’s winter range (i.e., not inclusive of the northern Yucatan, southern portions of Jamaica, and Puerto Rico) likely because of the species’ furtive behavior. Not surprisingly, poor occurrence data on the wintering grounds still limit our current understanding of their winter distribution. However, our study highlights how critical the use of tracking technology is in furthering our knowledge about elusive species.
The FLP we observed on both spring and fall migration, representative of prolonged over-water migrations while crossing the Gulf of Mexico/Caribbean Sea, allowed us to estimate both the likely routes taken during migration and migratory timing. Because all five Louisiana individuals demonstrated diurnal migration in the fall and spring, we can be more confident that they all made Gulf crossings and did not take a longer, less risky route along the coast of Mexico (Deppe et al. 2015, Adamík et al. 2016, Ward et al. 2018). Moreover, our most conservative estimate of flight duration for fall and spring migration was 23.72 and 19.2 hours respectively, which is similar to the 22 hours approximated for fall gulf crossings of three songbird species in Deppe et al. (2015). In the spring, two of the three Jamaica individuals did not demonstrate any diurnal movements, but all three took direct diurnal flights in the fall, averaging 21.86 hours in duration, two hours less than Louisiana individuals. Given the strong selective pressure to minimize time spent in spring migration to facilitate early arrival to the breeding grounds (Nilsson et al. 2013, Schmaljohann 2018), we would expect birds to make a more direct crossing in spring. One possibility might be that, during fall migration, birds encounter more favorable conditions, e.g., tailwinds (Dossman et al. 2016), for a direct flight, even flying directly from the Atlantic or Gulf Coast and surpassing Florida altogether (DeLuca et al. 2015, La Sorte et al. 2016). However, the prevalence of these over-water crossings suggests that Swainson’s Warblers in both the fall and spring require critical refueling sites on either side of the Gulf of Mexico and Caribbean portions of the Atlantic Ocean.
The time frame in which these prolonged diurnal over-water flights took place further represents conservative estimates of overall migration timing, with one notable observation being the Jamaica birds that migrated three weeks later than Louisiana conspecifics in the fall. When augmenting the FLP data with the automated telemetry data in Jamaica we were able to quantify the timing of migration more effectively for this population of Swainson’s Warblers and found an interesting bimodal relationship in the timing of spring migration. The birds that departed from Jamaica considerably earlier in the season did so within a similar time frame to the Louisiana individuals and, further, traveled on a more northwestern trajectory than the birds that departed later in the season. Given the trajectory of these earlier migrating individuals, it is likely they bred in the central part of the Swainson’s Warbler breeding range (Alabama, Mississippi, Tennessee), which, therefore, could explain why their migratory movements are earlier and more similar to Louisiana conspecifics. Another potential explanation of this bimodal relationship in departure is that the later-departing individuals are females, because males would depart earlier to arrive to their breeding grounds sooner. We cannot definitively test this, however, because all the birds captured during the breeding season in Louisiana were males, and we did not collect any blood from the Jamaica individuals to evaluate sex. This pattern is most likely driven by phenological differences in the timing of breeding at these more southerly breeding latitudes. In fact, many species of migrants generally demonstrate an intraspecific latitudinal gradient in migration phenology with northern breeding individuals tending to migrate later in the season than their southern breeding counterparts (Cohen et al. 2019).
We determined that Swainson’s Warblers migrated at an approximate rate of 200 km / d, similar to previously reported estimates of comparably sized passerines (Nilsson et al. 2013, Schmaljohann and Both 2017). However, our estimates of migration rate were quite variable and were as fast as 465 km / d. These faster rates were likely accomplished by direct over-water crossings rather than shorter flights punctuated by stopovers in Cuba, the Bahamas, and Florida. In fact, estimates of migration rate from geolocator tracks of the Louisiana birds migrating in the spring over the Gulf of Mexico were comparable to the fastest migration rates from the automated telemetry data of birds migrating from Jamaica through Florida. Such differences in phenology and migratory behaviors (routes and rates) likely further point to population differences (Bennett et al. 2019) evident within this nonbreeding population in Jamaica and may likely serve as a potential mechanism maintaining genetic differentiation across the breeding range. Understanding how migratory strategies vary across populations and influence population dynamics will require a full assessment of migratory connectivity of this population, coupled with finer resolution tracking approaches, either through refinements in current approaches (expansion of Motus tracking network or light-level geolocators) or advances in battery technology that result in further miniaturization of GPS tracking technology.
As in many studies that rely on data from archival tags, our research was limited by data quantity and quality (Bridge et al. 2011). One of our original goals was to determine whether light-level geolocation was even possible for a species like the Swainson’s Warbler that uses very dense habitat on both their breeding and wintering range. Geolocators are useful for coarse tracking, but light-level geolocation for relatively fine-scale information on migratory pathways is likely not an option for this and similar species that occupy understory habitats. However, because options to track small birds (< 20 g) outside the Motus Wildlife Tracking System are presently still limited to geolocators, researchers may wish to maximize information obtainable even from apparently poor light data. In this study, we leveraged instances of FLP to quantify the timing and approximate migratory routes, but even differences in the degree of shading could, arguably, illuminate other interesting biological differences among individuals and populations. For example, compared with the birds tagged in Jamaica, we have noticed that light data coinciding with the Central American and Mexican wintering grounds were distinctly worse (shadier) for the birds tagged in Louisiana. Although we did not investigate this more closely in this study, this pattern may reflect differences in habitat cover, e.g., darker humid forests of Central America versus more open, dry forests of the Caribbean.
Overall, our results suggest that Swainson’s Warblers maintain strong migratory connectivity with an east/west migratory divide that only further reinforces the moderate longitudinal population structure (Winker and Graves 2008). However, a more widespread coordinated deployment is needed to generate quantitative estimates of connectivity, e.g., MC metric (Cohen et al. 2018). Furthering this evidence of population separation and genetic differentiation are the differences in migratory behaviors, with the average timing of both fall and spring migration varying by one to three weeks. We are intrigued by the prevalence of diurnal migratory movements, which highlights the frequency of direct over-water crossings during both spring and fall migration and the potential consequences of these movements at the individual level. Each of these facets are novel discoveries for Swainson’s Warblers, yet we acknowledge that much more research needs to be conducted on each of these aspects to elucidate our understanding of all phases within their annual cycle. For instance, we might observe variable population responses to regionally specific changes in climate and rates of habitat loss, given possible strong migratory connectivity and variable east/west population trends. Further, because Swainson’s Warblers are of high conservation concern with already low estimated population numbers, the species would benefit from efforts targeting populations known to be at a relatively higher risk across their annual cycle.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.ACKNOWLEDGMENTS
We thank the many research technicians and volunteers for their dedicated assistance in the field. Thank you to the Petroleum Corporation of Jamaica (PCJ) for access to the field site at Font Hill Nature Preserve and to the Jamaican National Environmental Planning Agency (NEPA) for providing permits for conducting research. The Recreation and Park Commission for the Parish of East Baton Rouge (BREC) facilitated access to research sites in Louisiana. We also thank our fellow researchers for setting up and maintaining receiver stations across the Motus Wildlife Tracking System. This research was supported by funds from the Ohio Agricultural Research and Development Center (C. M. Tonra), Cornell Lab of Ornithology Athena Fund (B. C. Dossman), National Science Foundation (#0717338 and #1242584; P. P. Marra), SI James Bond Fund (B. C. Dossman and P. P. Marra), Baton Rouge Audubon Society (E. I. Johnson), American Ornithological Society, Wilson Ornithological Society, and National Institute of Food and Agriculture, U.S. Department of Agriculture, McIntire Stennis project no. 94327 (V. Jirinec). The manuscript was approved by the Director of the Louisiana State University Agricultural Center as manuscript number 2022-241-37257. All applicable institutional and/or national guidelines for the care and use of animals were followed. The authors declare that they have no conflict of interest.
DATA AVAILABILITY
The data/code that support the findings of this study will be available on the Dryad data repository (Brunner 2022) before publication of this manuscript.
LITERATURE CITED
Adamík, P., T. Emmenegger, M. Briedis, L. Gustafsson, I. Henshaw, M. Krist, T. Laaksonen, F. Liechti, P. Procházka, V. Salewski, et al. 2016. Barrier crossing in small avian migrants: individual tracking reveals prolonged nocturnal flights into the day as a common migratory strategy. Scientific Reports 6:21560. https://doi.org/10.1038/srep21560
Anich, N. M., T. J. Benson, J. D. Brown, C. Roa, J. C. Bednarz, R. E. Brown, and J. G. Dickson. 2020. Swainson’s Warbler (Limnothlypis swainsonii), version 1.0. In P. G. Rodewald, editor. Birds of the world. Cornell Lab of Ornithology, Ithaca, New York, USA. https://doi.org/10.2173/bow.swawar.01
Askins, R. A. 2000. Restoring North America’s birds: lessons from landscape ecology. Yale University Press, New Haven, Connecticut, USA. https://doi.org/10.12987/9780300127119
Bennett, R. E., A. D. Rodewald, K. V. Rosenberg, R. Chandler, L. Chavarria-Duriaux, J. A. Gerwin, D. I. King, and J. L. Larkin. 2019. Drivers of variation in migration behavior for a linked population of long-distance migratory passerine. Auk 136:1-13. https://doi.org/10.1093/auk/ukz051
Bridge, E. S., K. Thorup, M. S. Bowlin, P. B. Chilson, R. H. Diehl, R. W. Fléron, P. Hartl, R. Kays, J. F. Kelly, W. D. Robinson, et al. 2011. Technology on the move: recent and forthcoming innovations for tracking migratory birds. BioScience 61(9):689-698. https://doi.org/10.1525/bio.2011.61.9.7
Brlík, V., J. Koleček, M. Burgess, S. Hahn, D. Humple, M. Krist, J. Ouwehand, E. L. Weiser, P. Adamík, J. A. Alves, et al. 2020. Weak effects of geolocators on small birds: a meta-analysis controlled for phylogeny and publication bias. Journal of Animal Ecology 89(1):207-220. https://doi.org/10.1111/1365-2656.12962
Brunner, A. R, C. M. Tonra, and P. P. Marra 2022. Vulnerable Neotropical migratory songbird demonstrates flexibility in space use in response to rainfall change. Ornithology 139(2):1-14. https://doi.org/10.1093/ornithology/ukac005
Brzustowski, J., and D. Lepage. 2021. Motus: fetch and use data from the Motus Wildlife Tracking System, R Package Version 4.0.6. Bird Studies Canada, Port Rowan, Ontario, Canada. https://motus.org/
Cohen, E. B., J. A. Hostetler, M. T. Hallworth, C. S. Rushing, T. S. Sillett and P. P. Marra. 2018. Quantifying the strength of migratory connectivity. Methods in Ecology and Evolution, 9:513-524. https://doi.org/10.1111/2041-210X.12916
Cohen, E. B., C. R. Rushing, F. R. Moore, M. T. Hallworth, J. A. Hostetler, M. G. Ramirez, and P. P. Marra. 2019. The strength of migratory connectivity for birds en route to breeding through the Gulf of Mexico. Ecography 42:658-669. https://doi.org/10.1111/ecog.03974
Crawford, R. L. 1980. Wind direction and the species composition of autumn TV tower kills in northwest Florida. Auk 97:892-895.
Culp, L. A., E. B. Cohen, A. L. Scarpignato, W. E. Thogmartin, and P. P. Marra. 2017. Full annual cycle climate change vulnerability assessment for migratory birds. Ecosphere 8(3):e01565. https://doi.org/10.1002/ecs2.1565
DeLuca, W. V., B. K. Woodworth., C. C. Rimmer, P. P. Marra, P. D. Taylor, K. P. McFarland, S. A. Mackenzie, and D. R. Norris. 2015. Transoceanic migration by a 12-g songbird. Biology Letters 11(4):20141045. https://doi.org/10.1098/rsbl.2014.1045
Deppe, J. L., M. P. Ward, R. T. Bolus, R. H. Diehl, A. Celis-Murillo, T. J. Zenzal, F. R. Moore, T. J. Benson, J. A. Smolinsky, L. N. Schofield, et al. 2015. Fat, weather, and date affect migratory songbirds’ departure decisions, routes, and time it takes to cross the Gulf of Mexico. Proceedings of the National Academy of Sciences 112(46):E6331-E6338. https://doi.org/10.1073/pnas.1503381112
Dokter, A. M., A. Farnsworth, D. Fink, V. Ruiz-Gutierrez, W. M. Hochachka, F. A. La Sorte, O. J. Robinson, K. V. Rosenberg, and S. Kelling. 2018. Seasonal abundance and survival of North America’s migratory avifauna determined by weather radar. Nature Ecology and Evolution 2(10):1603-1609. https://doi.org/10.1038/s41559-018-0666-4
Dossman, B. C., G. W. Mitchell, D. R. Norris, P. D. Taylor, C. G. Guglielmo, S. N. Matthews, and P. G. Rodewald. 2016. The effects of wind and fuel stores on stopover departure behavior across a migratory barrier. Behavioral Ecology 27(2):567-574. https://doi.org/10.1093/beheco/arv189
Faaborg, J., R. T. Holmes, A. D. Anders, K. L. Bildstein, K. M. Dugger, S. A. Gauthreaux, Jr., P. Heglund, K. A. Hobson, A. E. Jahn, D. H. Johnson, et al. 2010. Recent advances in understanding migration systems of New World land birds. Ecological Monographs 80(1):3-48. https://doi.org/10.1890/09-0395.1
Fuller, M. R., W. S. Seegar, and L. S. Schueck. 1998. Routes and travel rates of migrating Peregrine Falcons Falco peregrinus and Swainson’s Hawks Buteo swainsoni in the Western Hemisphere. Journal of Avian Biology 29(4):433-440. https://doi.org/10.2307/3677162
Goldstein, M. I., T. E. Lacher, B. Woodbridge, M. J. Bechard, S. B. Canavelli, M. E. Zaccagnini, G. P. Cobb, E. J. Scollon, R. Tribolet, and M. J. Hooper. 1999. Monocrotophos-induced mass mortality of Swainson’s Hawks in Argentina, 1995-96. Ecotoxicology 8:201-214. https://doi.org/10.1023/A:1026496331396
Hahn, S., V. Amrhein, P. Zehtindijev, and F. Liechti. 2013. Strong migratory connectivity and seasonally shifting isotopic niches in geographically separated populations of a long-distance migrating songbird. Oecologia 173(4):1217-1225. https://doi.org/10.1007/s00442-013-2726-4
Hallworth, M. T., E. Bayne, E. McKinnon, O. Love, J. A. Tremblay, B. Drolet, J. Ibarzabal, S. Van Wilgenburg, and P. P. Marra. 2021. Habitat loss on the breeding grounds is a major contributor to population declines in a long-distance migratory songbird. Proceedings of the Royal Society B 288(1949):20203164. https://doi.org/10.1098/rspb.2020.3164
Hallworth, M. T., T. S. Sillett, S. L. Van Wilgenburg, K. A. Hobson, and P. P. Marra. 2015. Migratory connectivity of a Neotropical migratory songbird revealed by archival light-level geolocators. Ecological Applications 25(2):336-47. https://doi.org/10.1890/14-0195.1
Hewson, C. M., K. Thorup, J. W. Pearce-Higgins, and P. W. Atkinson. 2016. Population decline is linked to migration route in the Common Cuckoo. Nature Communications 7:12296. https://doi.org/10.1038/ncomms12296
Kays, R., M. C. Crofoot, W. Jetz, and M. Wikelski. 2015. Terrestrial animal tracking as an eye on life and planet. Science 348:6240. https://doi.org/10.1126/science.aaa2478
Kramer, G. R., D. E. Anderson, D. A. Buehler, P. B. Wood, S. M. Peterson, J. A. Lehman, K. R. Aldinger, L. P. Bullock, S. Harding, J. A. Jones, et al. 2018. Population trends in Vermivora warblers are linked to strong migratory connectivity. Proceedings of the National Academy of Sciences 115(14):E3192-E3200.
La Sorte, F. A., D. Fink, W. M. Hochachka, and S. Kelling. 2016. Convergence of broad-scale migration strategies in terrestrial birds. Proceedings of the Royal Society B 283(1823):20152588. https://doi.org/10.1098/rspb.2015.2588
Lisovski, S., S. Bauer, M. Briedis, S. C. Davidson, K. L. Dhanjal-adams, M. T. Hallworth, J. Karagicheva, C. M. Meier, B. Merkel, J. Ouwehand, l. Pedersen, et al. 2020. Light-level geolocator analyses: a user’s guide. Journal of Animal Ecology 89(1):221-236. https://doi.org/10.1111/1365-2656.13036
MacDonald, C., K. Fraser, H. Gilchrist, T. Kyser, J. Fox, and O. Love. 2012. Strong migratory connectivity in a declining arctic passerine. Animal Migration 1(1):23-30. https://doi.org/10.2478/ami-2012-0003
Marra, P. P., E. B. Cohen, S. R. Loss, J. E. Rutter, and C. M. Tonra. 2015. A call for full annual cycle research in animal ecology. Biology Letters 11(8):20150552. https://doi.org/10.1098/rsbl.2015.0552
Marra, P. P., D. Hunter, and A. M. Perrault. Migratory connectivity and the conservation of migratory animals. 2011. Enviromental Law 41:317-354.
Marra, P. P., D. R. Norris, S. M. Haig, M. S. Webster, and J. A. Royle. 2006. Migratory connectivity. Pages 157-183 in K. R. Crooks and M. Sanjayan, editors. Connectivity conservation. Cambridge University Press, New York, New York, USA. https://doi.org/10.1017/CBO9780511754821.008
McKinnon, E. A., and O. P. Love. 2018. Ten years tracking the migrations of small landbirds: lessons learned in the golden age of bio-logging. Auk 135(4):834-56. https://doi.org/10.1642/AUK-17-202.1
Meanley, B. 1971. Natural history of the Swainson’s Warbler. North American Fauna 69, Bureau of Sport, Fisheries, and Wildlife, United States Department of the Interior, Washington, D.C., USA. https://doi.org/10.3996/nafa.69.0001
Mitchell, G. W., A. E. M. Newman, M. Wikelski, and D. R. Norris. 2012. Timing of breeding carries over to influence migratory departure in a songbird: an automated radiotracking study. Journal of Animal Ecology 81(5):1024-1033. https://doi.org/10.1111/j.1365-2656.2012.01978.x
Nilsson, C., R. H. Klaassen, and T. Alerstam. 2013. Differences in speed and duration of bird migration between spring and autumn. American Naturalist 181(6):837-845. https://doi.org/10.1086/670335
Oliveira, S. L., D. J. Flaspohler, J. L. Knowlton, C. R. Webster, and J. D. Wolfe. 2021. Migratory bird community structure in oil palm (Elaies guineensis) plantations and native forest fragments in southern Mexico. Journal of Field Ornithology 92(1):1-17. https://doi.org/10.1111/jofo.12354
Rappole, J. H., and A. R. Tipton. 1991. New harness design for attachment of radio transmitters to small passerines. Journal of Field Ornithology 62(3):335-337.
Rosenberg, K. V., A. M. Dokter, P. J. Blancher, J. R. Sauer, A. C. Smith, P. A. Smith, J. C. Stanton, A. Panjabi, L. Helft, M. Parr, and P. P. Marra. 2019. Decline of North American avifauna. Science 366(6461):120-124. https://doi.org/10.1126/science.aaw1313
Rosenberg, K. V., J. A. Kennedy, R. Dettmers, R. P. Ford, D. Reynolds, J. D. Alexander, C. J. Beardmore, P. J. Blancher, R. E. Bogart, G. S. Butcher, A. F. Camfield, et al. 2016. Partners in Flight landbird conservation plan: 2016 revision for Canada and Continental United States. Partners in Flight Science Committee, U.S. Fish and Wildlife Service, Washington, D.C., USA.
Rushing, C. S., J. A. Hostetler, T. S. Sillett, P. P. Marra, J. A. Rotenberg, and T. B. Ryder. 2017. Spatial and temporal drivers of avian population dynamics across the annual cycle. Ecology 98(11):2837-2850. https://doi.org/10.1002/ecy.1967
Sauer, J. R., D. K. Niven, J. E. Hines, D. J. Ziolkowski, Jr., K. L. Pardieck, J. E. Fallon, and W. A. Link. 2017. The North American breeding bird survey, results and analysis 1966–2015. Version 2.07.2017, United States Geological Survey Patuxent Wildlife Research Center, Laurel, Maryland, USA.
Schmaljohann, H. 2018. Proximate mechanisms affecting seasonal differences in migration speed of avian species. Scientific Reports 8:4106. https://doi.org/10.1038/s41598-018-22421-7
Schmaljohann, H., and C. Both. 2017. The limits of modifying migration speed to adjust to climate change. Nature Climate Change 7:573-576. https://doi.org/10.1038/nclimate3336
Sheehy, J., C. M. Taylor, K. S. McCann, and D. R. Norris. 2010. Optimal conservation planning for migratory animals: integrating demographic information across seasons. Conservation Letters 3(3):192-202. https://doi.org/10.1111/j.1755-263X.2010.00100.x
Sillett, T. S., and R. T. Holmes. 2002. Variation in survivorship of a migratory songbird throughout its annual cycle. Journal of Animal Ecology 71(2):296-308. https://doi.org/10.1046/j.1365-2656.2002.00599.x
Simons, T. R., F. R. Moore, and S. A. Gauthreaux. 2004. Mist netting trans-Gulf migrants at coastal stopover sites: the influence of spatial and temporal variability on capture data. Studies in Avian Biology 29:135-143.
Stevenson, H. M., and B. H. Anderson. 1994. The birdlife of Florida. University Press of Florida, Gainesville, Florida, USA.
Sullivan, B. L., C. L. Wood, M. J. Iliff, R. E. Bonney, D. Fink, and S. Kelling. 2009. eBird: a citizen-based bird observation network in the biological sciences. Biological Conservation 142(10):2282-2292. https://doi.org/10.1016/j.biocon.2009.05.006
Taylor, W. K., and B. H. Anderson. 1973. Nocturnal migrants killed at a central Florida TV tower: autumns 1969–1971. Wilson Bulletin 85(1):42-51.
Taylor, P. D., T. L. Crewe, S. A. Mackenzie, D. Lepage, Y. Aubry, Z. Crysler, G. Finney, C. M. Francis, C. G. Guglielmo, D. J. Hamilton, et al. 2017. The Motus Wildlife Tracking System: a collaborative research network to enhance the understanding of wildlife movement. Avian Conservation and Ecology 12(1):8. https://doi.org/10.5751/ACE-00953-120108
Taylor, W. K., and M. A. Kershner. 1986. Migrant birds killed at the vehicle assembly building (VAB), John F. Kennedy Space Center. Journal of Field Ornithology 57(2):142-154.
Tonra, C. M., M. T. Hallworth, T. J. Boves, J. Reese, L. P. Bullock, M. Johnson, C. Viverette, K. Percy, E. M. Ames, A, Matthews, M. C. Slevin, et al. 2019. Concentration of a widespread breeding population in a few critically important nonbreeding areas: migratory connectivity in the Prothonotary Warbler. Condor 121:1-15. https://doi.org/10.1093/condor/duz019
Twedt, D. J., and C. R. Loesch. 2001. Forest area and distribution in the Mississippi Alluvial Valley: implications for breeding bird conservation. Journal of Biogeography 26(6):1215-1224. https://doi.org/10.1046/j.1365-2699.1999.00348.x
U.S. Fish and Wildlife Service. 2008. Birds of conservation concern 2008. U.S. Department of Interior, Fish and Wildlife Service, Division of Migratory Bird Management, Arlington, Virginia, USA. http://www.fws.gov/migratorybirds/
Ward, M. P., T. J. Benson, J. Deppe, T. J. Zenzal, R. H. Diehl, A. Celis-Murillo, R. Bolus, and F. R. Moore. 2018. Estimating apparent survival of songbirds crossing the Gulf of Mexico during autumn migration. Proceedings of the Royal Society B 285:20181747. https://doi.org/10.1098/rspb.2018.1747
Webster, M. S., P. P. Marra, S. M. Haig, S. Bensch, and R. T. Holmes. 2002. Links between worlds: unraveling migratory connectivity. Trends in Ecology and Evolution 17(2):76-83. https://doi.org/10.1016/S0169-5347(01)02380-1
Wickham, H., M. Averick, J. Bryan, W. Chang, L. D. A. Mcgowan, R. François, G. Grolemund, A. Hayes, L. Henry, J. Hester, et al. 2019. Welcome to the Tidyverse. Journal of Open Source Software 4(43):1686. https://doi.org/10.21105/joss.01686
Willson, J. D., and C. T. Winne. 2016. Evaluating the functional importance of secretive species: a case study of aquatic snake predators in isolated wetlands. Journal of Zoology 298(4):266-273. https://doi.org/10.1111/jzo.12311
Winker, K., and G. R. Graves. 2008. Genetic structure of breeding and wintering populations of Swainson’s Warbler. The Wilson Journal of Ornithology 120(3):433-445. https://doi.org/10.1676/07-073.1
Fig. 1

Fig. 1. Example of a diurnal movement (15–16 September 2018) during fall migration in the light data of a light-level geolocator deployed on an individual Swainson’s Warbler (JAM774) on 3 March 2018 at Font Hill, Jamaica.

Fig. 2

Fig. 2. Estimated breeding and wintering sites (mean ± SE) of Swainson’s Warblers tagged during the winter period at the Font Hill Nature preserve in southwest Jamaica or tagged in Louisiana during the breeding season. Dashed lines connect tagging locations (black circle) with estimated breeding or wintering sites (gray triangles).

Fig. 3

Fig. 3. Spring migratory routes (A) of radio-tagged Swainson’s Warblers wintering at the Font Hill Nature Preserve in southwest Jamaica and detected upon departure from Jamaica and detected en route on the Motus Wildlife Tracking Array in Florida, Georgia, and North Carolina. Spring departure schedules (black) and timing of detections of birds in Florida (gray) during migration (B). Distribution of individual spring migration rates (C) traveling between Jamaica and the southeastern U.S.

Table 1
Table 1. Geolocator estimates of breeding and wintering sites of birds tagged either during the breeding season in Louisiana or during the winter in Jamaica in 2018. Estimates of migration timing were derived from uninterrupted light intensity data indicative of a diurnal migratory movement over the Gulf of Mexico or Caribbean Sea.
| Bird ID | Age | Site | Date Deployed | Mean Winter Longitude | Mean Winter Latitude | Mean Breeding Longitude | Mean Breeding Latitude | Spring Diurnal Migration | Fall Diurnal Migration |
| JAM773 | SY | Font Hill | 23-Jan | -77.94201 | 18.04282 | -81.60348 | 30.93001 | Apr 10–11 | Sept 14–15 |
| JAM774 | SY | Font Hill | 3-Mar | -77.94201 | 18.04282 | -84.52548 | 31.84538 | None | Sept 15–16 |
| JAM777 | ASY | Font Hill | 11-Mar | -77.94201 | 18.04282 | -78.69761 | 36.24209 | None | Sept 20–21 |
| LA705 | ASY | Frenchtown | 28-Jun | -87.88974 | 19.65227 | -90.99074 | 30.47887 | Apr 13–14 | Oct 3–4 |
| LA706 | ASY | Palmetto | 6-Jul | -84.14549 | 22.21337 | -92.14483 | 29.86568 | Mar 30–31 | Oct 11–12 |
| LA708 | ASY | Frenchtown | 22-Jun | -88.47657 | 19.60934 | -90.98119 | 30.47654 | Mar 30–31 | Sept 17–18 |
| LA710 | ASY | Palmetto | 2-Jul | -91.95131 | 16.78122 | -92.13435 | 29.86873 | Apr 4–5 | Oct 11–12 |
| LA711 | ASY | Frenchtown | 20-Jun | -91.48055 | 18.44245 | -90.98826 | 30.47265 | Mar 24–25 | Oct 11–12 |
Table 2
Table 2. Spring departure date from Font Hill, Jamaica, and spring migration detections on the Motus Wildlife Tracking array in Florida, Georgia, and North Carolina. Data presented from nanotags deployed on Swainson’s Warblers at Font Hill Nature Preserve, Jamaica in 2016, 2017 and 2019.
| Year | Bird ID | Age | Date Deployed | Spring Departure | Date of Migration Detection | Detection Location |
| 2016 | 70 | AHY | 15-Apr-16 | 26-Apr-16 | 18-May-16 | Cedar Island NWR, NC |
| 2016 | 72 | AHY | 14-Apr-16 | 24-Apr-16 | NA | NA |
| 2017 | 514 | ASY | 18-Mar-17 | 18-Apr-17 | NA | NA |
| 2017 | 513 | ASY | 20-Mar-17 | 18-Apr-17 | NA | NA |
| 2017 | 515 | ASY | 27-Mar-17 | 1-Apr-17 | 15-Apr-17 | Brunswick, GA |
| 2017 | 517 | ASY | 28-Mar-17 | 31-Mar-17 | 3-Apr-17 | St. Marks NWR, FL |
| 2017 | 512 | ASY | 29-Mar-17 | 2-Apr-17 | NA | NA |
| 2017 | 521 | ASY | 30-Mar-17 | 4-Apr-17 | 16-Apr-17 | Brunswick, GA |
| 2017 | 518 | ASY | 26-Mar-17 | 6-Apr-17 | NA | NA |
| 2017 | 519 | ASY | 12-Apr-17 | 18-Apr-17 | 27-Apr-17 | Faver-Dykes SP, FL |
| 2017 | 516 | ASY | 20-Mar-17 | 5-Apr-17 | 11-Apr-17 | Dunn's Creek SP, FL |
| 2017 | 520 | ASY | 28-Mar-17 | 20-Apr-17 | 28-Apr-17 | Brunswick, GA |
| 2019 | 60 | ASY | 16-Feb-19 | 6-Apr-19 | 18-Apr-19 | Little Talbot SP, FL |
| 2019 | 59 | SY | 18-Mar-19 | 6-Apr-19 | 18-Apr-19 | Little Talbot SP, FL |
| 2019 | 61 | SY | 2-Apr-19 | 1-May-19 | 4-May-19 | Florida Panther NWR, FL |
| 2019 | 62 | ASY | 20-Mar-19 | 19-Apr-19 | 26-Apr-19 | Washington Oaks SP, FL |
| 2019 | 317 | SY | 25-Mar-19 | 14-Apr-19 | 19-Apr-19 | Washington Oaks SP, FL |