The following is the established format for referencing this article:
Mejias, M. A., and B. Misiuk. 2025. Territoriality and site fidelity of an island endemic subspecies, the Bermuda White-eyed Vireo (Vireo griseus bermudianus). Journal of Field Ornithology 96(1):4.ABSTRACT
Territories are areas that contain resources and are occupied by animals that defend these areas from conspecifics and heterospecifics. Territoriality in birds, which use vocal displays to repel intruders from their territories, has been thoroughly studied in many taxa, including the Bermuda White-eyed Vireo (Vireo griseus bermudianus). However, it remains unclear how long this subspecies holds a territory, whether territory occupancy differs between sexes, and how large defended areas are. We explored these questions by observing color-banded birds from 2016 to 2023. Some (23%) Bermuda Vireo pairs held the same territories for at least one year. Although the amount of time sexes spent within a woodland patch did not appear to differ (P = 0.08), males remained faithful to the same specific territories longer than females (P < 0.01). Among neighboring vireos, GPS observations of mated pairs showed more spatial overlap than coordinates for vireos that were not breeding with one another (P < 0.01). The average space utilized by bermudianus was 4156 ± 2315 m² (0.42 ha) according to the 90% utilization distribution. Our study concludes that Bermuda Vireos strongly defend small, year-round territories from consubspecifics, and sheds light on how Bermuda Vireos utilize wooded space. Our findings should aid in conservation management for this endemic subspecies and could assist other conservationists studying vireonids beyond Bermuda.
RESUMEN
Los territorios son áreas que contienen recursos y son ocupadas por animales que defienden estas áreas de conespecíficos y heteroespecíficos. La territorialidad en aves, la cual utiliza despliegues vocales para repeler a intrusos de sus territorios, ha sido estudiada profundamente en muchos taxa, incluyendo el Verderón Ojiblanco de Bermuda (Vireo griseus bermudianus). Sin embargo, todavía no está claro cuánto tiempo esta subespecie mantiene un territorio, si la ocupación del territorio difiere entre sexos, y tampoco cuán grandes son las áreas defendidas. Hemos explorado estas preguntas mediante la observación de individuos con anillos de colores, desde 2016 hasta 2023. Algunas parejas de verderones (23%) mantuvieron los mismos territorios por al menos un año. Aunque el tiempo que cada sexo pasó en un parche de bosque no pareció diferir (P = 0.08), los machos se mantuvieron fieles a un mismo territorio específico por más tiempo que las hembras (P < 0.01). Entre verderones vecinos, observaciones de GPS de parejas apareadas mostraron un mayor solapamiento espacial que las coordenadas para verderones que no se estaban reproduciendo entre ellos (P < 0.01). El espacio promedio utilizado por bermudianus fue de 4156 ± 2315 m² (0.42 ha) de acuerdo al 90% de distribución de uso. Nuestro estudio concluye que los Verderones de Bermuda defienden fuertemente territorios pequeños de conespecíficos todo el año, y arroja luz sobre cómo los Verderones de Bermuda utilizan el espacio boscoso. Nuestros hallazgos deberían ayudar en el manejo en conservación para esta subespecie endémica, y podrían asistir a otros conservacionistas que estudian vireónidos más allá de Bermuda.
INTRODUCTION
Within the context of behavioral ecology, a territory is an area where an animal lives and uses behavior to exclude conspecifics and heterospecifics from the defended area (Brown and Orians 1970, Hinsch and Komedeur 2017). Examples of territorial behavior include howling and scent-marking in Ethiopian wolves (Canis simensis; Sillero-Zubiri and Macdonald 1998), visual displays by mantis shrimp (Neogonodactylus bredini; Green and Patek 2018), and tree scarring by moose (Alces alces; Argunov 2021). Several hypotheses suggest that individual fitness may increase as a function of resources that come with defending and holding a territory. These resources include food (Justino et al. 2012), shelter (Taborsky et al. 2014), mates (Hasegawa et al. 2012), and areas in which to raise young (Pfeiffer and Meyburg 2015). Competition for these resources, usually between a territory “owner” and an “intruder,” forms the basis of the territoriality framework (Hinsch and Komedeur 2017, Gutiérrez-Carrillo et al. 2023). While animal behaviorists have debated the semantics of this framework (Hinde 1956, Kamath and Wesner 2020), long-term field studies of marked individuals that show territory fidelity and exclusion of neighboring conspecifics, coupled with explicitly defined terms, are essential for improving our understanding of animal territoriality.
The vocal displays of songbirds are thought to be a territorial behavior that advertises the occupancy of utilized space (Kroodsma and Byers 1991, Mejías and Wilson 2023a). For example, in an intervention experiment, Krebs (1977) removed male Great Tits (Parus major) from their territories during the breeding season and replaced them with a speaker broadcasting either their species song or a silent control and found higher rates of conspecific intrusions into vacant territories during silent controls than conspecific song playbacks. Migratory songbirds that breed in temperate regions are known to sing tirelessly within their northern breeding grounds but do not sing after migrating to their southern non-breeding grounds. Presumably, migrant songbirds do not sing within their non-breeding grounds because they are not actively defending breeding territories. In contrast, non-migratory tropical songbird species sing year-round inside their territories (Diamond 1974, Slater and Mann 2004). Although there is ample evidence of territoriality in several songbird species, its prevalence and strength remain unclear in other avian taxa, such as those living on remote, oceanic islands.
Bermuda is home to a vociferous, non-migratory, endemic subspecies of passerine, the Bermuda White-eyed Vireo (Vireo griseus bermudianus; hereafter, “Bermuda Vireo”). Following permanent human settlement on the island in the 17th century, the indigenous woodlands have been replaced almost entirely with introduced flora, and virtually all endemic landbird species became extinct, except for the Bermuda Vireo, which has been assigned the highest level of protection status in Bermuda (Wingate 1990, Mejías 2021). Despite a proliferation of invasive species and a loss of other landbirds, both single birds and mated Bermuda Vireo pairs remain common in woodlands across Bermuda year-round (Mejías and Nol 2020, Mejías 2021). Bermuda Vireos breed between February and September (Mejías and Wilson 2023b), and although the local population size of bermudianus has yet to be estimated, birdwatching observations since 2011 (M. A. Mejías, personal observation) and ongoing vireo banding efforts suggests the population is stable at a conservative estimate of at least 2000 individuals, with many vireos readily found in nearly all wooded habitats across Bermuda (Mejías 2021). Although this subspecies defends territories from consubspecifics and heterospecifics with physical and vocal displays (Mejías et al. 2021), it remains unclear how long these vireos hold their territories, whether mated males and females exhibit differences in territory occupancy, the size of areas utilized by bachelor males and breeding pairs, and the extent to which these spaces overlap.
Here we explore the territorial behavior of uniquely, color-banded Bermuda Vireos to address these knowledge gaps. Our first objective is to determine whether Bermuda Vireos demonstrate year-round territory fidelity. Second, we investigate whether territories are occupied longer by males or females by comparing the number of days either sex is observed within a territory. Third, we compare overlaps in space use to test field observations that bachelor males and breeding pairs defend exclusive territories, and to determine whether clusters of neighboring territories overlap in area. The degree of overlap in space use amongst neighboring vireos is explored as a proxy for the strength of territoriality in this subspecies; if strong territoriality is present, space use by neighboring vireos within areas of suitable habitat would show little to no overlap. Finally, we estimate the sizes of areas that Bermuda Vireos inhabit at their territories in two-dimensional space. Understanding the total habitat area that Bermuda Vireos use, and the duration of use, not only informs local conservation management decisions for this protected songbird, but additionally may have relevance to research outside Bermuda that explores vireo conservation and habitat management.
METHODS
Study sites and vireo observations
Bermuda is a remote archipelago in the Northwest Atlantic (32°18′N, 64°47′W) with aeoline limestone terrain that is low-lying, albeit hilly (range: 0–76 m, mean: 38 m) and is subtropical in climate (18–27.5 °C). During the spring and summer months (March–September), sunshine and light winds dominate, whereas the fall and winter (October–February) may have strong winds and gales (Amos 1991). Twenty-first century wooded habitat is dominated by exotic introduced trees, many of which are considered invasive; indigenous flora are far less common (Mejías and Nol 2020). Our 14 study sites in Bermuda (Fig. 1) contained largely invasive species, including allspice (Pimenta dioica), Brazilian pepper (Schinus terebinthifolius), casuarina (Casuarina equisetifolia), fiddlewood (Citharexylum spinosum), and Chinese fan palm (Livistona chinensis), and a few native and endemic trees such as Bermuda palmetto (Sabal bermudana), Bermuda cedar (Juniperus bermudiana), southern hackberry (Celtis laevigata), and bay grape (Coccoloba uvifera).
Bermuda Vireo mark-recapture efforts were initiated by local birdwatcher P. Watson in 2015, and were continued and expanded to an independent study of color-banded Bermuda Vireos in 2016 by M. A. Mejías, including research on singing and breeding biology. From June 2017 to November 2020, we captured Bermuda Vireos using mist nets along walking trails and clearings across 14 study sites, which included sites where P. Watson began banding birds in 2015 (Fig. 1). Given previous observations suggesting strong territoriality (Mejías et al. 2021), we lured vireos using consubspecific song recordings. We sexed Bermuda Vireos as they approached mist nets, or during subsequent visits to territories by confirming whether they sang discrete or rambling songs, both of which are used only by males (Bradley 1981, Mejías and Wilson 2023a). In contrast, female responses to playback were weaker than males, resulting in fewer female captures. We fitted and released all netted vireos with a uniquely numbered magnesium-aluminium alloy Porzana band on one leg, and either one or two Darvic color bands on one or both legs for individual identification from afar (Fig. 2). Total handling time was ≤ 15 min per bird.
From August 2016 to May 2021, across the breeding and non-breeding seasons, we used binoculars and a handheld GPS to record sightings of color-banded Bermuda Vireos. Locations of nest sites and perches where birds commonly vocalized (i.e., singing and/or producing scolding calls; Mejías et al. 2021) were recorded, often opportunistically, using a handheld GPS unit (Garmin eTrex® 10), including observations of birds banded previously by P. Watson. However, locational data with the GPS was collected incorrectly throughout 2016–2017. Therefore, we limited our analyses to GPS data from May 2018 to May 2021. Following previous studies on avian habitat use, all GPS locations, per individual, were taken at least 15 min apart to reduce autocorrelation between observations (Mazerolle and Hobson 2004). Nonetheless, previous work has indicated that estimates of the total area used by marked individuals become increasingly accurate as the number of points increases, even though autocorrelation also increases (Swihart and Slade 1985). Although GPS points associated with vireo sightings were no longer collected after May 2021, we continued to record field encounters of color-banded vireos during birdwatching outings until October 2023 to quantify how long birds and their banded mates occupied territories. Per Mejías and Wilson (2023b), we considered vireos to be paired if color-banded males and females were seen occupying the same space over the duration of the study period, and these pair bonds to have ended if one of the color-banded vireos was no longer observed associating with its previous mate and its territory. We provide estimates of the duration (in days) in which males and females were each observed within their respective study sites and territories for birds whose territories we visited regularly and mapped. The duration of study site occupancy was estimated by calculating the total number of days from the date color-banded vireos were first captured and banded in the study site, to the last date they were seen inside their respective territories. Similarly, we estimated territory occupancy by calculating the total amount of days color-banded vireos were first and last seen within their territories. We suggest that these estimates are conservative; occupancy of both study site and territories likely occurred before and after our monitoring period of color-banded vireos. The durations for which vireos occupied the same general study site (Fig. 1), and also the same territory, were quantified and compared between males and females using two sample t-tests. We considered differences between sexes to be statistically significant if P < 0.05.
Territory mapping and analysis
We explored vireo territoriality using estimates of space use from year-round field sightings of color-banded birds, including perches of vocalizing vireos and branches with active nests during the breeding seasons. A number of techniques exist by which to estimate the space use of an animal, but comparative studies suggest that use of the Utilization Distribution (UD) is a robust solution, particularly using kernel density methods (Worton 1989, Börger et al. 2006, Lichti and Swihart 2011). The UD is defined as the two-dimensional frequency distribution for observation locations of an animal over time (van Winkle 1975). In other words, the UD is a probability density that an individual will be found at a given location, based on fixed-point observation data collected from that individual across time. A critical decision when calculating the kernel density for the UD is the selection of an appropriate bandwidth parameter. Here, bandwidths were selected according to the “plug-in” method (Wand and Jones 1994), which appears to perform comparatively well for estimating space use (Gitzen et al. 2006). Plug-in bandwidth selection was performed using the R package “ks” (Duong 2007), which supports two-dimensional unconstrained (i.e., non-diagonal) bandwidth estimation, allowing for off-axis kernel orientations. Thus, UDs were calculated using the R package “adehabitatHR” (Calenge 2006) with two-dimensional kernel estimation from “ks”.
The UDs of different animals can be compared to observe patterns of joint space use. Here, we used the Utilization Distribution Overlap Index (UDOI) as a metric of space-use sharing between birds that overlapped temporally, throughout the breeding and non-breeding period, across multiple years (Fieberg and Kochanny 2005). UDOI values of 1 indicate two distributions that are uniform in space (i.e., not clustered) with 100% overlap. Values > 1 indicate two non-uniform distributions that have a high degree of overlap, for example, where individuals appear to preferentially utilize the same space. Values < 1 suggest non-uniform distributions with low overlap. In the context of this study, lower UDOI values therefore suggest that two birds increasingly avoid use of the same space, potentially exhibiting territoriality.
Field observations suggest that Bermuda Vireos may exhibit territoriality, and that mating pairs may share a territory (Mejías et al. 2021). The UDOI was first used to test whether mating vireo pairs inhabit the same areas. This was accomplished by identifying every pair of birds sharing overlapping UDs and calculating their UDOIs. We defined “patches” as areas of continuous and open woodland at a study site inhabited by one or more individuals. The extent of each patch was determined using the union of the maximum UD estimates for each individual (the 99.9th percentile). In other words, a “patch” is considered to contain one or multiple individuals where their UDs may be connected through a union of other UDs. Then, at each patch, the UDOI was calculated between the UD of each pair of mating birds and each pair of non-mating birds to determine whether birds within the same local patches tend to use the same space, or whether they deter one another, exhibiting territoriality. Distributions of UDOI values were observed to be non-normal; UDOIs were compared between mating and non-mating pairs with > 3 observations using the Wilcoxon rank-sum test. Results were considered significant if P < 0.05.
Having calculated the UDs as a probability density, it is possible to estimate the space use of each male or mating pair according to the probability of observing the individual in space. Here, the 90% contour was calculated for each male or mating pair using their UD, which corresponds to the smallest area in which the probability of observing the individual is 0.90. The 90% UD contour provides a compromise between the accuracy and precision of space use estimates, and may also exhibit less bias compared to higher values (e.g., 95–100%; Börger et al. 2006). Estimated 90% UD sizes were regressed against the number of vireo observations per territory to test whether the sample size for individuals or pairs may have biased the estimated extent of their space use.
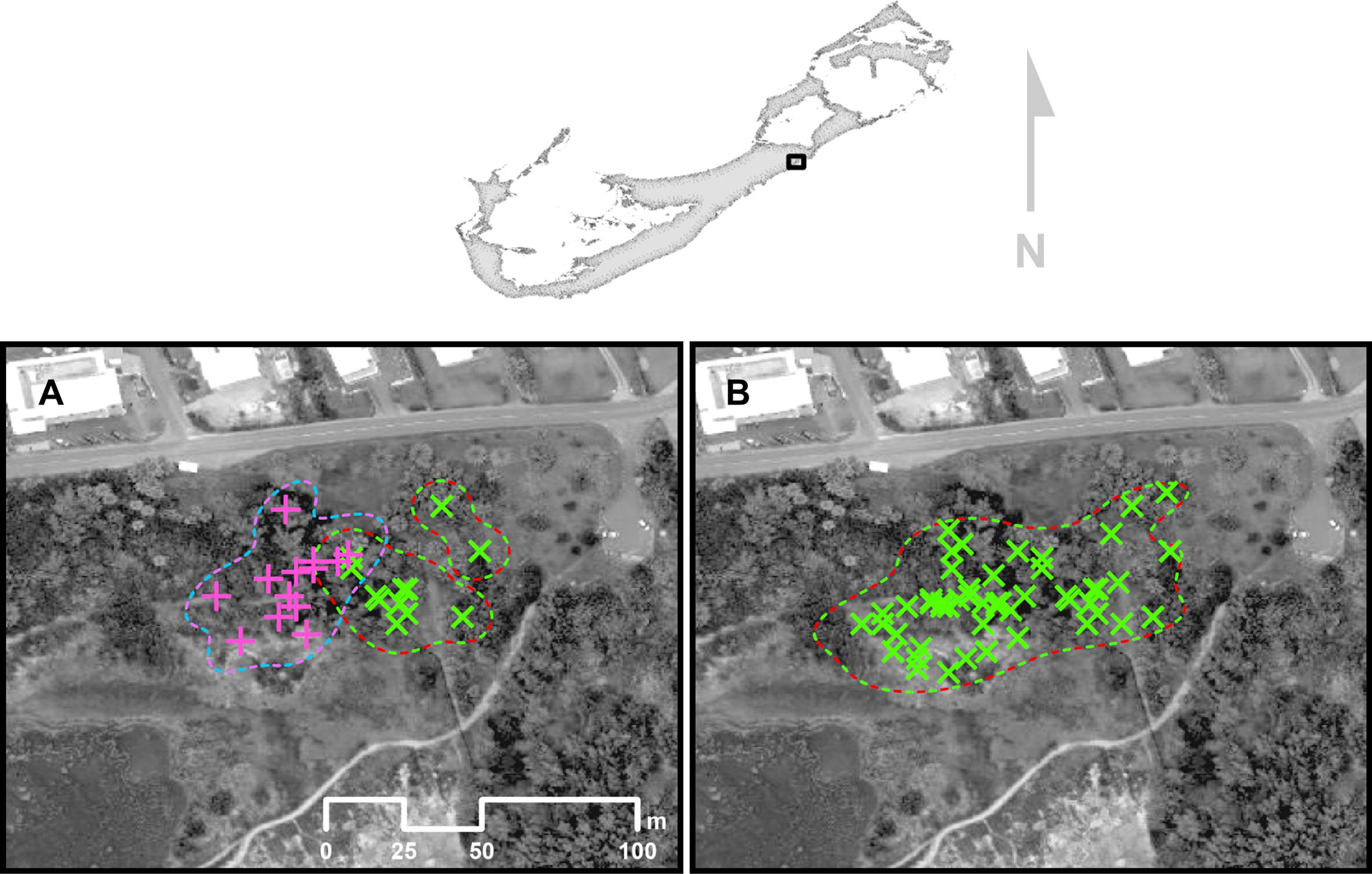
A territory takeover event was recorded during field observations in 2019. A male bird (banded right leg [R]: GreenRed and left leg [L]: Orange; hereafter referred to as “GreenRed”; Fig. 2) began to utilize territory previously occupied by a neighboring male (R: PurpleBlue L: Orange; hereafter “PurpleBlue”). GreenRed continued to occupy this area throughout field observations and was sighted 38 more times. PurpleBlue was not sighted again within the area that it originally occupied. To document and measure this apparent exchange of territory, space use was estimated for both GreenRed and PurpleBlue prior to the event, and the territory size of GreenRed was additionally estimated after. The 90% UD was calculated and compared before and after the takeover.
RESULTS
Study population, behavior, and site fidelity
Between 2017 and 2019, M. A. Mejías color-banded 106 Bermuda Vireos across the 14 study sites. Throughout our study period, we collected resighting data from 100 color-banded vireos: 59 (59%) of these were banded by the main author and 41 (41%) by P. Watson. Based on the vocal behavior of our 100 vireos, we sexed 85 individuals: 66 males and 19 females. Breeding pairs were commonly seen shadowing one another closely as they moved through their territories. The loud and frequent songs of males made them much easier to detect than females, which do not sing. This resulted in more GPS coordinates recorded for males (N = 1175) than females (108). We identified 22 vireo pairs amongst our color-banded birds. Among the 22 pairs, 5 (23%) were confirmed occupying the same territory for at least one year (i.e., ≥ 365 days; Table 1). Although we found no significant difference in the duration that respective patches (i.e., locations in Fig. 1) were occupied by males (mean = 856.02, SD = 733.79 days) and females (mean = 553.95, SD = 535.07 days), t(46.79) = -1.81, P = 0.08, we found that, on average, a single given territory was occupied longer by male vireos (mean = 662.55,SD = 773.54 days) than females (mean = 239.45,SD = 377.36 days), t(61.81) = -2.93, P < 0.01 (Table 1).
Territoriality and mating
From 2016 to 2021, we collected a total of 1332 GPS coordinates for 100 color-banded vireos. Because GPS data from the first two years were not reliable, 785 points (59%) from 2018–2021, taken from 47 Bermuda Vireos, were used to map 37 territories across the archipelago. The average number of GPS points, per territory, was N = 21 (min = 5, max = 50, SD = 14). Space use estimates between vireos within a given patch suggested that non-mating birds tend to avoid use of the same space (Fig. 3). A low median UDOI value (0.01) indicated that non-mating birds were normally seldom observed at overlapping areas within a single continuous patch, though we noted the presence of several outliers (Fig. 4). The median UDOI for mating pairs (0.49) was significantly higher according to the Wilcoxon rank sum test (P < 0.01), suggesting that mating pairs commonly occupied the same space. These results concur with field observations suggesting territoriality between non-mating vireos, and shared territories between mating pairs.
Given that vireos appear to exhibit territoriality, UDs were also used to characterize the areal extent that bachelor males or breeding pairs defend. The average 90% UD was 4156 ± 2315 m² for males or male-female mating pairs (Fig. 5a). We found no significant relationship between the number of individual observations and the 90% contour size (P = 0.83, adjusted R² = -0.03), indicating that these estimates were not biased by the observation prevalence (Fig. 5b).
Prior to the territory takeover event observed on 26 April 2019, there was little overlap observed between areas occupied by GreenRed and PurpleBlue (Fig. 6A). The area occupied by GreenRed was estimated to be ~1673 m² according to the 90% UD; the area occupied by PurpleBlue was estimated to be ~1926 m². After the takeover, PurpleBlue was not observed again in this vicinity. For the duration of the study, GreenRed was observed inhabiting portions of the territory previously occupied by PurpleBlue, in addition to its original territory (Fig. 6B). The new area utilized by GreenRed, including observations obtained after 26 April was ~4082 m², suggesting a more than two-fold increase in space occupied after the takeover event (i.e., 144% increase in the 90% UD).
DISCUSSION
We used uniquely colored leg bands to study woodland space use by the Bermuda Vireo, V. griseus bermudianus. We first showed that individuals utilized the same woodland patches year-round, and that both bachelor males and male-female pairs, the latter of which were commonly seen travelling close together, defended exclusive territories inside their respective woodlands. Vireo territories were small, and exclusion between neighbors, especially males, was fierce. One territorial conflict was observed wherein a male entered a neighboring territory and physically forced the neighboring male out, increasing its own territory size.
Although male and female vireos were faithful to the same woodland sites year-round, male birds remained inside the same year-round territories longer than females. Their annual presence is not surprising because bermudianus is non-migratory (Mejías and Wilson 2023b), exhibiting a sedentary lifestyle like tropical passerines (Hau and Beebe 2011, Budka et al. 2023). However, annual fidelity to breeding territories has also been recorded in migrant vireos, including Red-eyed Vireo (V. olivaceus), Warbling Vireo (V. gilvus), and Bell’s Vireo (V. bellii; Pletschet 1987, Joos et al. 2014). A similar banding study on migratory, White-eyed Vireos in Virginia found that males arrived significantly earlier to breeding sites than females and nearly all males were faithful to the previous season’s territory, whereas females were often re-sighted in adjacent territories (Hopp et al. 1999). These findings are congruent with our observations of resident Bermuda Vireos. Specifically, we commonly observed male Bermuda Vireos inside the same territories year-round, but these males would often be seen paired with a different female during subsequent field observations, within and between years (Table 1). In 2017, Mejías and Wilson (2023b) reported one color-banded female forming sequentially monogamous pair bonds with four neighboring color-banded males during the same breeding season. Using a sexual selection framework (Andersson 1994), our collective results suggest White-eyed Vireos have a breeding system in which males, the competing sex, own and defend resources within annual territories, whereas females, the choosy sex, are only associated with a given territory through their current pair bond with a male, and are free to desert at any time.
Bermuda Vireos occupied distinct spaces with minimal overlap. Space use boundaries were generally well defined, with territories occupied by either a bachelor male or a male-female pair, with little to no intrusions by neighboring birds. White-eyed Vireos are known to repel conspecifics by patrolling their territories, singing their primary species song, used only by males, or using scolding or chatter calls, performed by both sexes (Bradley 1980, 1981). Mejías et al. 2021 found that male Bermuda Vireos produced more vocalizations and speaker flyovers when consubspecific recordings were played in the center of the territory of the signaler. Under natural conditions, if their vocal displays fail, territoriality escalates to physical confrontation, with vireos exchanging midair pecks, wing slaps, and foot grappling on the edges of adjacent territories (Bradley 1980; M. A. Mejías, personal observation). Bermuda Vireos seem to reserve their aggression toward consubspecifics; we never observed physical altercations with sympatric Northern Cardinals (Cardinalis cardinalis) or Gray Catbirds (Dumetella carolinensis), larger songbirds whose own breeding territories often overlapped with Bermuda Vireos. Interspecific competition among mainland vireos is well documented, and studies often hypothesize that the strength of conflict is due to the extent of similarity in foraging style, prey items, and habitat and microhabitat type (Robinson and Holmes 1982). Sympatric, Red-eyed Vireos and Philadelphia Vireos (V. philadelphicus) react strongly to one another’s songs and defend territories from conspecific and heterospecific intruders (Rice 1978), whereas the songs and presence of Red-eyed and Blue-headed Vireos (V. solitarius) with overlapping territories elicited little aggression from either vireonid (Hudman and Chandler 2002). Our space use analyses provide evidence for the former scenario, with Bermuda Vireos exhibiting strong consubspecific competition for the same resources, effectively repelling one another.
Bermuda Vireo two-dimensional space use is small. The average area (90% contour) occupied by territorial vireos was 4156 m² (i.e., ~0.42 ha), similar to the 0.5-hectare territories held by White-eyed Vireos wintering in the Yucatan Peninsula (Greenberg et al. 1993). Breeding territory size estimates for other V. griseus populations are currently lacking. Several North American vireonids have similar-sized breeding territories to bermudianus, like the Philadelphia Vireo (3088 m²; 0.31 ha; Rice 1978) and Red-eyed Vireo (3900 m²; 0.39 ha; Marshall and Cooper 2004), although larger areas have been defended by Black-capped Vireos (V. atricapilla; 20,000 m², 2.0 ha; Colon 2016). In general, temperate songbirds defend much smaller territories than their tropical counterparts, with some tropical species holding average territories as big as 13 ha (Stouffer 2007). Therefore, the small areas occupied by Bermuda Vireos likely reflects their ancestry to North American White-eyed Vireos (Mejías et al. 2021). Despite the apparent smaller territory sizes of White-eyed Vireos, the average territorial space use observed here for the Bermuda Vireo (4156 m²) seems to be optimal for this subspecies’ survival. Only one Bermuda Vireo pair was found on Gamma Island (an area ~8000 m², ~0.8 ha), and their territory was confined to the south-east corner of the island (90% UD = 2654 m²,0.3 ha; Fig. 3C), even though ample space was available and there was no consubspecific competition. This suggests that Bermuda Vireos naturally hold small territories. In our study, one color-banded male increased his territory size through a takeover, yielding an occupied area much closer to the sample population average (from 1673 m² to 4082 m²). This was accomplished by entering his neighbor’s territory and singing while pursued, branch to branch, by the original territory owner. The result was abandonment of the territory by the latter male soon thereafter. The victorious male made no further attempts to increase his territory size for the remainder of the study period, further emphasizing the limited area Bermuda Vireos defend. Our documentation of this increase in territory size, before and after a real-time territorial dispute, to our knowledge, is the first such published record for any vireonid in the literature and increases our understanding of the areal woodland habitat requirements for mate attraction, breeding, and survival.
CONCLUSION
The Bermuda Vireo is the last remaining endemic landbird on the island and is currently given the highest level of protection under the Bermuda law (Mejías 2021). The results of this study have increased our understanding of the length of residency and space use of Bermuda Vireos in woodlands across the archipelago. By the early 1980s, new housing units in Bermuda were being built at a rate of about 300 per year (Dobson 2002), resulting in about 14% of the island being converted into impenetrable surfaces. Despite this significant development, Bermuda Vireos remain common throughout the island, likely because of apparent small territory requirements observed here, and also their tendency to nest in both indigenous and introduced trees (Mejías and Wilson 2023b). Despite their resiliency, anthropogenic impacts such as continued woodland clearing are likely to negatively impact this endemic subspecies. Because male birds tend to remain faithful to their territories across years, they may be prone to remain inside territories that have been impacted by development and rendered suboptimal, which could reduce their ability to attract and maintain long lasting pair bonds with females. This is an avenue for future research. Similarly, future work should aim to obtain a higher sample size of locational data for female vireos, to verify our findings of shared territoriality between mated pairs. The tendency of Bermuda Vireos to establish year-round territories inside indigenous and introduced vegetation should deter landowners from completely removing woodlands comprising introduced vegetation. To minimize habitat loss for the non-migratory Bermuda Vireo during native woodland restoration efforts, we recommend cutting out small (i.e., 0.1 ha patches, at least 25 m apart) sections of invasive trees inside a woodland patch at a time. This small space may be replanted with young native species, while removing invasive saplings on subsequent revisits. Allowing newly planted native trees to grow before removing more 0.1 ha patches of invasives would enable continued access to mature trees for Bermuda Vireos, ensuring the two-dimensional space of their territories remains largely intact. Similar work on other vireonid species in countries beyond Bermuda will be beneficial for understanding the duration of territory occupancy and minimum territory sizes these songbirds require for breeding and survival, and might help guide future development decisions that can minimize disturbance to habitats used by the Vireonidae.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.
AUTHOR CONTRIBUTIONS
Miguel Mejías: Conceptualization; fieldwork; field methodology; data entry; writing and editing – first and final draft.
Benjamin Misiuk: Conceptualization; writing and editing – first and final draft; data entry; data and mapping analyses.
ACKNOWLEDGMENTS
We would like to thank P. Watson for training M. Mejías in the capturing and banding of songbirds, as well as M. Outerbridge, who issued Bermuda fieldwork permits. Special thanks to Patricia and Albert Mejías for their help in the field. We would also like to thank the private landowners and Bermuda Government wardens who allowed us to capture and monitor vireos on their respective properties. Finally, we are greatly indebted to the anonymous reviewers whose feedback greatly improved our manuscript.
DATA AVAILABILITY
The dataset for this manuscript is available upon request from either author. Ethical approval for this work was granted by a research permit issued by the Bermuda Department of Environment and Natural Resources (License 17-02-02-59).
LITERATURE CITED
Amos, E. 1991. A guide to the birds of Bermuda. Warwick, Bermuda.
Andersson, M. 1994. Sexual selection (Vol. 72). Princeton University Press, Princeton, New Jersey, USA.
Argunov, A. V. 2021. Marking activities of the moose (Alces alces) in Central Yakutia. Biology Bulletin 48:1394-1400. https://doi.org/10.1134/S1062359021080033
Börger, L., N. Franconi, G. De Michele, A. Gantz, F. Meschi, A. Manica, S. Lovari, and T. Coulson. 2006. Effects of sampling regime on the mean and variance of home range size estimates. Journal of Animal Ecology 75:1393-1405. https://doi.org/10.1111/j.1365-2656.2006.01164.x
Bradley, R. A. 1980. Vocal and territorial behavior in the White-eyed Vireo. Wilson Bulletin 92:302-311.
Bradley, R. A. 1981. Song variation within a population of White-eyed Vireos (Vireo griseus). Auk 98:80-87. https://doi.org/10.1093/auk/98.1.80
Brown, J. L., and G. H. Orians. 1970. Spacing patterns in mobile animals. Annual Review of Ecology and Systematics 1:239-262. https://doi.org/10.1146/annurev.es.01.110170.001323
Budka, M., J. E. Uyeme, and T. S. Osiejuk. 2023. Females occasionally create duets with males but they never sing solo-year-round singing behaviour in an Afrotropical songbird. Scientific Reports 13:11405. https://doi.org/10.1038/s41598-023-38552-5
Calenge, C. 2006. The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling 197:516-519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Colon, M. R. 2016. Effects of drought on Black-Capped Vireo habitat selection and reproduction. Dissertation. Texas A&M University, College Station, Texas, USA.
Diamond, A. W. 1974. Annual cycles in Jamaican forest birds. Journal of Zoology 173:277-301. https://doi.org/10.1111/j.1469-7998.1974.tb03138.x
Dobson, A. 2002. A birdwatching guide to Bermuda. Arlequin, Chelmsford, UK.
Duong, T. 2007. ks: Kernel density estimation and kernel discriminant analysis for multivariate data in R. Journal of Statistical Software 21:1-16. https://doi.org/10.18637/jss.v021.i07
Fieberg, J., and C. O. Kochanny. 2005. Quantifying home-range overlap: the importance of the utilization distribution. Journal of Wildlife Management 69:1346-1359. https://doi.org/10.2193/0022-541X(2005)69[1346:QHOTIO]2.0.CO;2
Gitzen, R. A., J. J. Millspaugh, and B. J. Kernohan. 2006. Bandwidth selection for fixed-kernel analysis of animal utilization distributions. Journal of Wildlife Management 70:1334-1344. https://doi.org/10.2193/0022-541X(2006)70[1334:BSFFAO]2.0.CO;2
Green, P. A., and S. N. Patek. 2018. Mutual assessment during ritualized fighting in mantis shrimp (Stomatopoda). Proceedings of the Royal Society B: Biological Sciences 285:20172542. https://doi.org/10.1098/rspb.2017.2542
Greenberg, R., D. K. Niven, S. Hopp, and C. Boone. 1993. Frugivory and coexistence in a resident and a migratory vireo on the Yucatan Peninsula. Condor 95:990-999. https://doi.org/10.2307/1369434
Gutiérrez-Carrillo, D. A., C. D. Cadena, J. Rodríguez-Fuentes, and J. E. Avendaño. 2023. Nasty neighbours in the Neotropics: seasonal variation in physical and vocal aggression in a montane forest songbird. Animal Behaviour 200:81-90. https://doi.org/10.1016/j.anbehav.2023.02.006
Hasegawa, M., E. Arai, M. Watanabe, and M. Nakamura. 2012. Female mate choice based on territory quality in Barn Swallows. Journal of Ethology 30:143-150. https://doi.org/10.1007/s10164-011-0307-8
Hau, M., and K. Beebe. 2011. Plastic endocrine regulation of year-round territorial aggression in tropical male spotted antbirds. General and Comparative Endocrinology 172:305-313. https://doi.org/10.1016/j.ygcen.2011.03.016
Hinde, A. 1956. The biological significance of the territories of birds. Ibis 98:340-369. https://doi.org/10.1111/j.1474-919X.1956.tb01419.x
Hinsch, M., and J. Komdeur. 2017. What do territory owners defend against? Proceedings of the Royal Society B: Biological Sciences 284:20162356. https://doi.org/10.1098/rspb.2016.2356
Hopp, S. L., A. Kirby, and C. A. Boone. 1999. Banding returns, arrival pattern, and site-fidelity of White-eyed Vireos. Wilson Bulletin 111:46-55.
Hudman, S. P., and C. R. Chandler. 2002. Spatial and habitat relationships of Red-eyed and Blue-headed Vireos in the southern Appalachians. Wilson Bulletin 114:227-234. https://doi.org/10.1676/0043-5643(2002)114[0227:SAHROR]2.0.CO;2
Joos, C. J., F. R. Thompson III, and J. Faaborg. 2014. The role of territory settlement, individual quality, and nesting initiation on productivity of Bell's Vireos Vireo bellii bellii. Journal of Avian Biology 45:584-590. https://doi.org/10.1111/jav.00400
Justino, D. G., P. K. Maruyama, and P. E. Oliveira. 2012. Floral resource availability and hummingbird territorial behaviour on a Neotropical savanna shrub. Journal of Ornithology 153:189-197. https://doi.org/10.1007/s10336-011-0726-x
Kamath, A., and A. B. Wesner. 2020. Animal territoriality, property and access: a collaborative exchange between animal behaviour and the social sciences. Animal Behaviour 164:233-239. https://doi.org/10.1016/j.anbehav.2019.12.009
Krebs, J. R. 1977. Song and territory in the Great Tit Parus major. Pages 47-62 in B. Stonehouse and C. Perrins, editors. Evolutionary ecology. Macmillan, London, UK. https://doi.org/10.1007/978-1-349-05226-4_6
Kroodsma, D. E. and B. E. Byers. 1991. The function(s) of bird song. American Zoologist 31:318-328. https://doi.org/10.1093/icb/31.2.318
Lichti, N. I., and R. K. Swihart. 2011. Estimating utilization distributions with kernel versus local convex hull methods. Journal of Wildlife Management 75:413-422. https://doi.org/10.1002/jwmg.48
Marshall, M. R., and R. J. Cooper. 2004. Territory size of a migratory songbird in response to caterpillar density and foliage structure. Ecology 85:432-445. https://doi.org/10.1890/02-0548
Mazerolle, D. F., and K. A. Hobson. 2004. Territory size and overlap in male Ovenbirds: contrasting a fragmented and contiguous boreal forest. Canadian Journal of Zoology 82:1774-1781. https://doi.org/10.1139/z04-175
Mejías, M. A. 2021. Management plan for white-eyed vireos (Vireo griseus bermudianus). Department of Environment and Natural Resources, Government of Bermuda. https://environment.bm/species-recovery-plans
Mejías, M. A. and E. Nol. 2020. Woodland size and vegetation effects on resident and non-resident woodland birds in Bermuda. Journal of Caribbean Ornithology 33:22-32. https://doi.org/10.55431/jco.2020.33.22-32
Mejías, M. A., J. Roncal, and D. R. Wilson. 2021. Territorial responses of male Bermuda White-eyed Vireos (Vireo griseus subsp. bermudianus) reflect phylogenetic similarity of intruders and acoustic similarity of their songs. Journal of Field Ornithology 92:431-449. https://doi.org/10.1111/jofo.12384
Mejías, M. A. and D. R. Wilson. 2023a. The relationships of breeding stage to daytime singing behaviour and song perch height in Bermuda white-eyed vireos (Vireo griseus bermudianus). Journal of Avian Biology e03116. https://doi.org/10.22541/au.167104128.81180033/v1
Mejías, M. A. and D. R. Wilson. 2023b. Breeding biology and nesting behavior of the endemic subspecies of White-eyed Vireo (Vireo griseus bermudianus) on the Bermuda archipelago. Journal of Field Ornithology 94:(3):1. https://doi.org/10.5751/JFO-00307-940301
Pfeiffer, T., and B. U. Meyburg. 2015. GPS tracking of Red Kites (Milvus milvus) reveals fledgling number is negatively correlated with home range size. Journal of Ornithology 156:963-975. https://doi.org/10.1007/s10336-015-1230-5
Pletschet, S. M. 1987. Habitat preferences and interspecific competition: Red-eyed and warbling vireos. Thesis. University of California, Santa Cruz, USA. https://scholarworks.umt.edu/cgi/viewcontent.cgi?article=8072&context=etd
Rice, J. 1978. Ecological relationships of two interspecifically territorial vireos. Ecology 59:526-538. https://doi.org/10.2307/1936583
Robinson, S. K., and R. T. Holmes. 1982. Foraging behavior of forest birds: the relationships among search tactics, diet, and habitat structure. Ecology 63:1918-1931. https://doi.org/10.2307/1940130
Sillero-Zubiri, C., and D. W. Macdonald. 1998. Scent-marking and territorial behaviour of Ethiopian wolves Canis simensis. Journal of Zoology 245:351-361. https://doi.org/10.1111/j.1469-7998.1998.tb00110.x
Slater, P. J., and N. I. Mann. 2004. Why do the females of many bird species sing in the tropics? Journal of Avian Biology 35:289-294. https://doi.org/10.1111/j.0908-8857.2004.03392.x
Stouffer, P. C. 2007. Density, territory size, and long-term spatial dynamics of a guild of terrestrial insectivorous birds near Manaus, Brazil. Auk 124:291-306. https://doi.org/10.1093/auk/124.1.291
Swihart, R. K., and N. A. Slade. 1985. Influence of sampling interval on estimates of home-range size. Journal of Wildlife Management 49:1019-1025. https://doi.org/10.2307/3801388
Taborsky, B., L. Guyer, and P. Demus. 2014. ‘Prudent habitat choice’: a novel mechanism of size-assortative mating. Journal of Evolutionary Biology 27:1217-1228. https://doi.org/10.1111/jeb.12398
van Winkle, W. 1975. Comparison of several probabilistic home-range models. Journal of Wildlife Management 39:118-123. https://doi.org/10.2307/3800474
Wand, M., and M. Jones. 1994. Multivariate plug-in bandwidth selection. Computational Statistics 9:97-116.
Wingate, D. B. 1990. The restoration of Nonsuch Island as a living museum of Bermuda’s precolonial terrestrial biome. Pages 133-149 in G. M. Woodwell, editor. The Earth in transition: patterns and processes of biotic impoverishment. Cambridge University Press, Cambridge, UK. https://doi.org/10.1017/CBO9780511529917.009
Worton, B. J. 1989. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164-168. https://doi.org/10.2307/1938423
Fig. 1
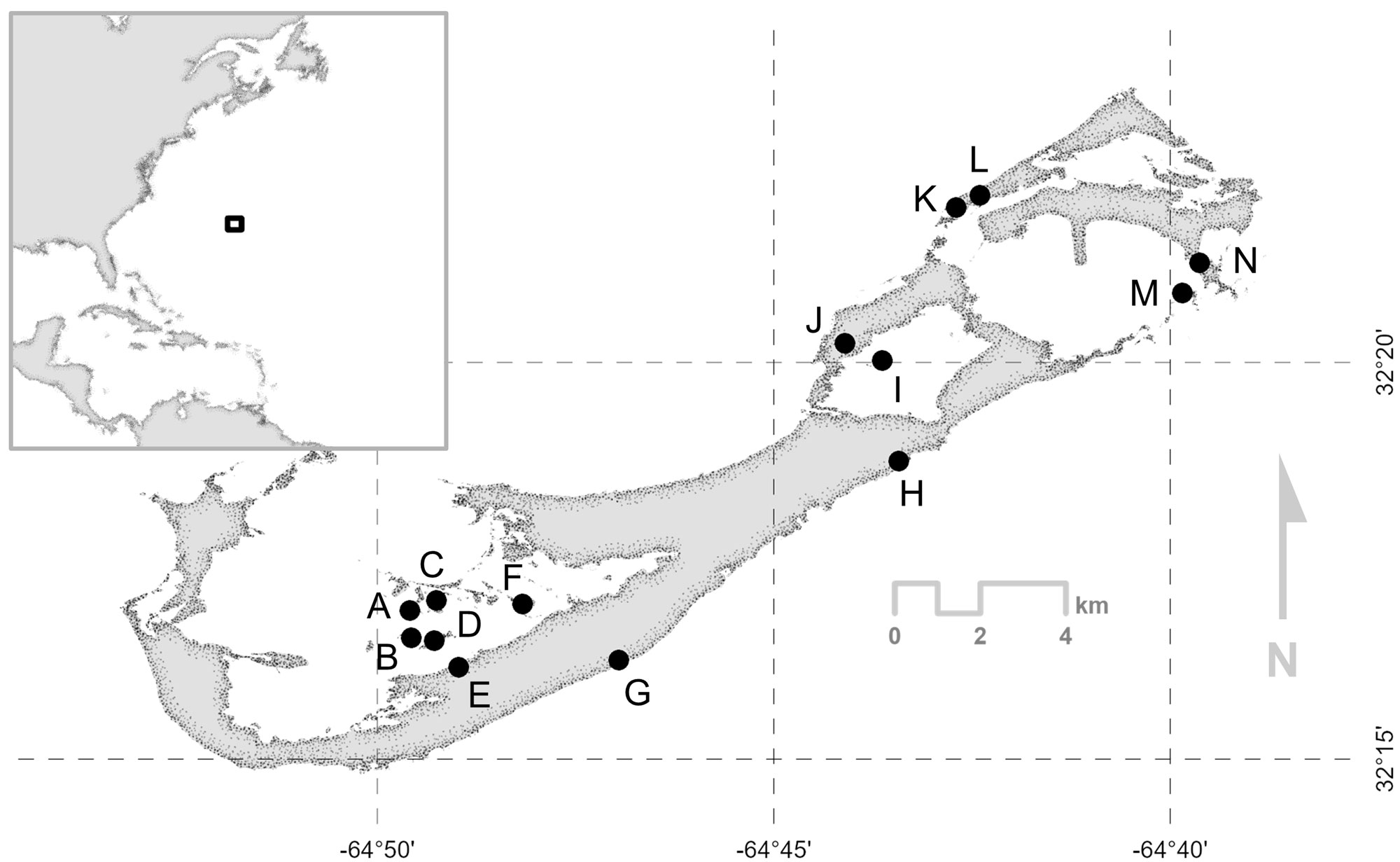
Fig. 2

Fig. 3
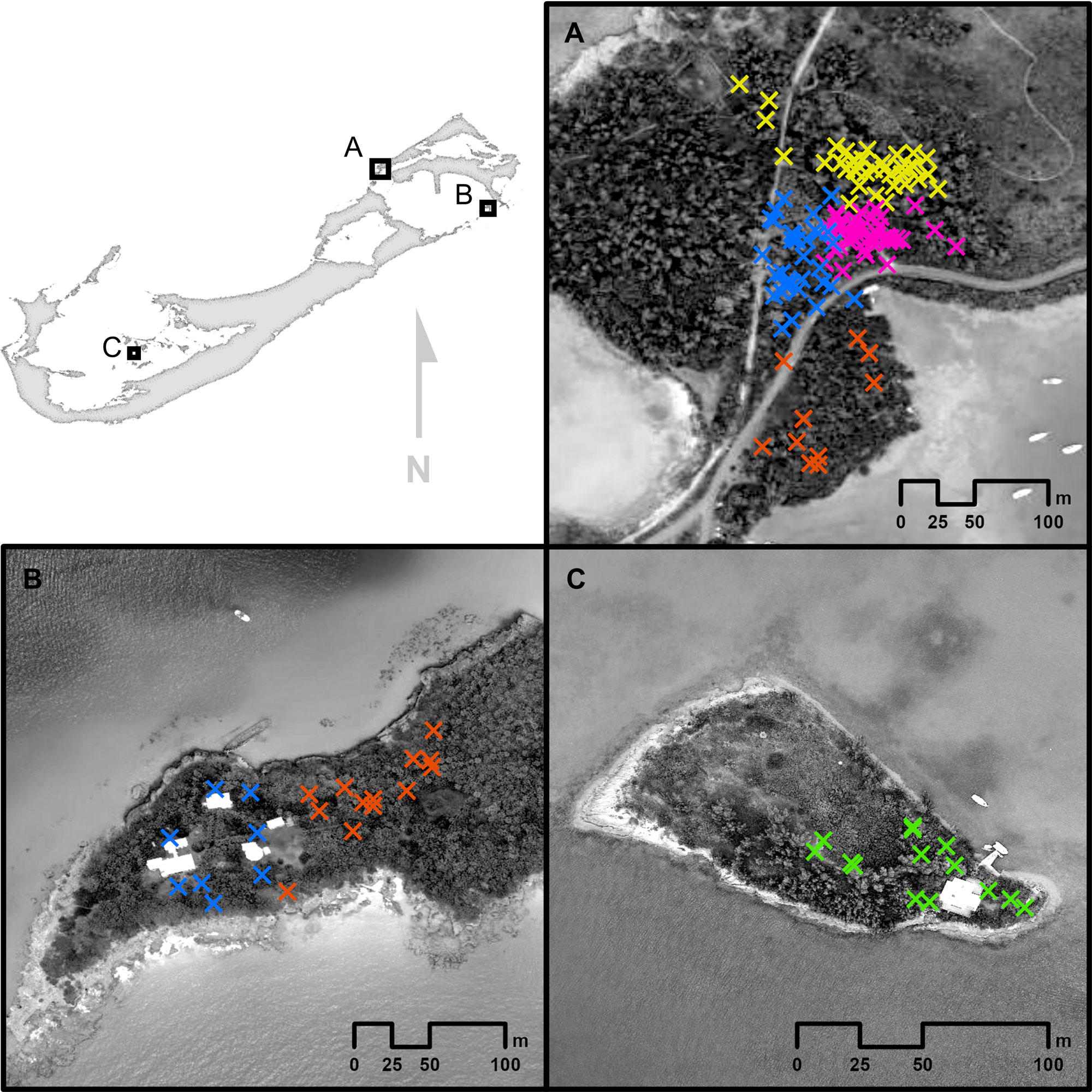
Fig. 4
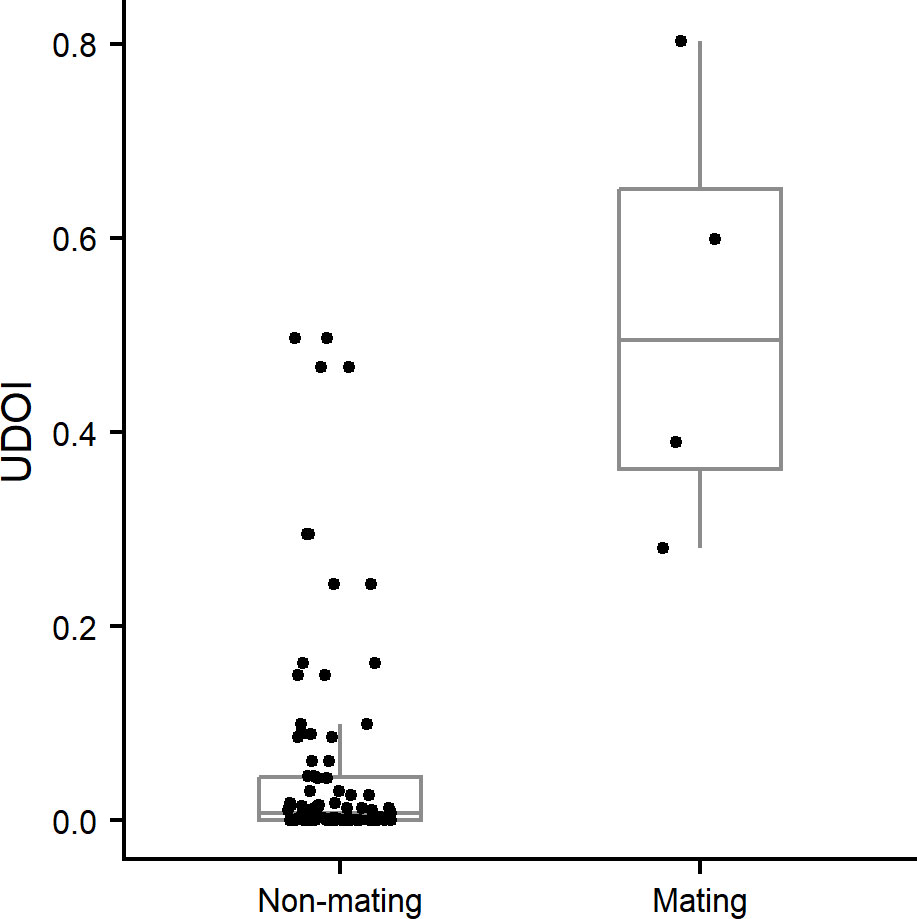
Fig. 5
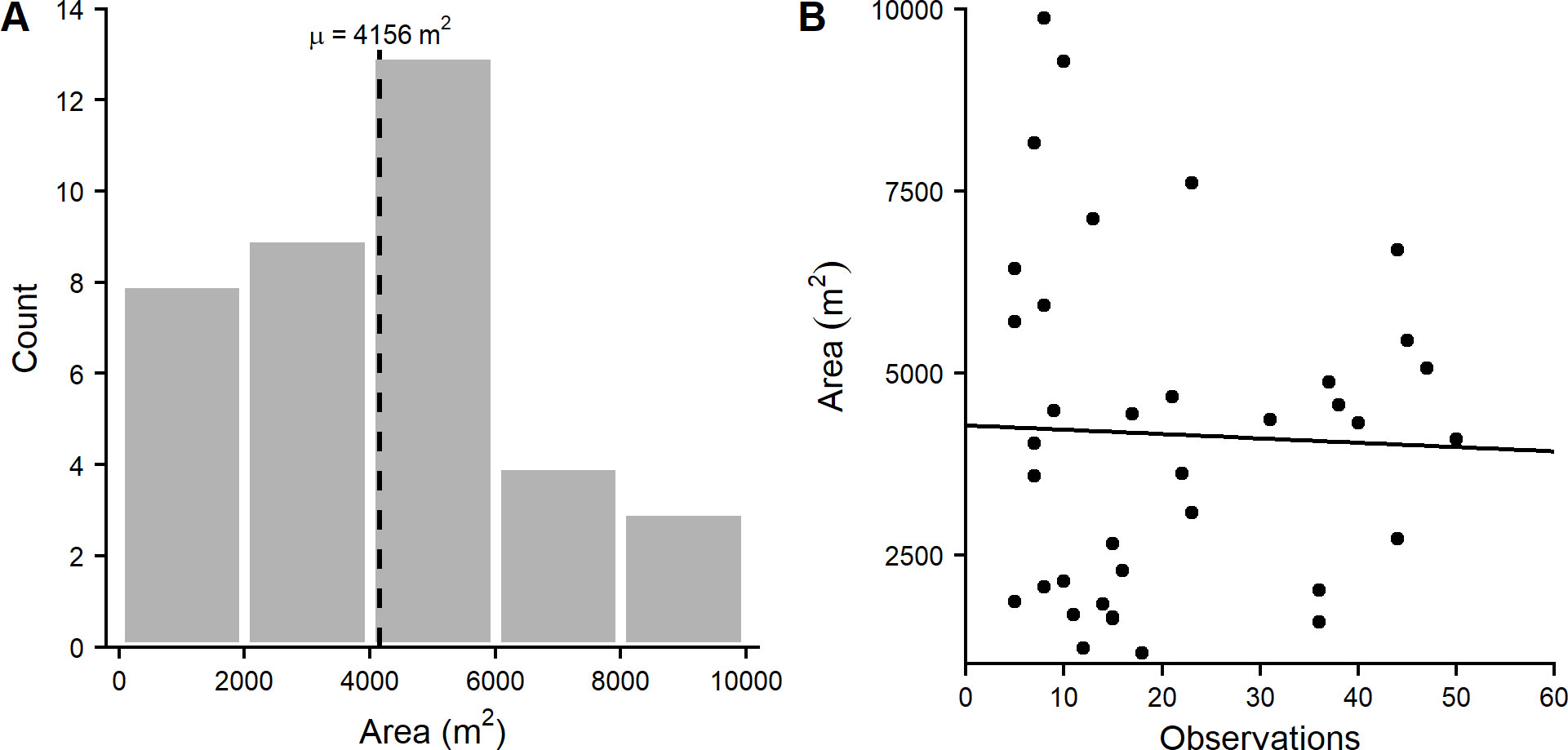
Fig. 6