The following is the established format for referencing this article:
Spicer, O. N., and E. A. Forys. 2023. Do larids exhibit sex-biased natal dispersal? A case study of a southwest Florida population of Black Skimmers (Rynchops niger). Journal of Field Ornithology 94(2):8.ABSTRACT
Understanding dispersal patterns in a species provides important evolutionary and ecological knowledge. Dispersal decreases inbreeding, which can greatly influence a species’ genetic diversity and conservation status. We examined natal dispersal sex differences in a southwest Florida population of Black Skimmers (Rynchops niger) by collecting band resighting data from 86 (57 males, 29 females) skimmers during the 2017–2022 breeding seasons. We examined sex biases in the proportion of natal dispersers and the distances traveled from their natal colonies. The majority of skimmers dispersed (72% of females, 61% of males), however, no statistically significant difference was found between the proportion or distance traveled between the sexes. As beach habitat becomes fragmented by development and sea level rise, the ability of both sexes to disperse may benefit both Black Skimmer genetics and demographics in a changing world.
RESUMEN
Comprender los patrones de dispersión de una especie proporciona importantes conocimientos evolutivos y ecológicos. La dispersión disminuye la endogamia, lo que puede influir enormemente en la diversidad genética y el estado de conservación de una especie. Examinamos las diferencias en la dispersión natal de cada sexo en una población del suroeste de Florida de Rayadores negros (Rynchops niger) mediante la recopilación de datos de reavistamiento de bandas de 86 individuos (57 machos, 29 hembras) durante las temporadas de cría 2017–2022. Examinamos los sesgos de sexo en la proporción de dispersores natales y las distancias recorridas desde sus colonias natales. La mayoría de los rayadores se dispersaron (72% de las hembras, 61% de los machos), sin embargo, no se encontraron diferencias estadísticamente significativas en la proporción o la distancia recorrida entre los sexos. A medida que el hábitat de la playa se fragmenta por el desarrollo y el aumento del nivel del mar, la capacidad de dispersión de ambos sexos puede beneficiar tanto a la genética como a la demografía del Rayador negro en un mundo cambiante.
INTRODUCTION
Studying philopatry and dispersal is vital for understanding the evolutionary biology, ecology, and conservation of birds. Natal philopatry describes the return of an individual to its natal site to breed (Coulson 2016, Antaky et al. 2021), although dispersal refers to either an individual’s movement between birth and their first reproductive event (natal dispersal) or the subsequent movement of an individual between breeding sites (breeding dispersal; Greenwood and Harvey 1982). Motivations for dispersal include the avoidance of inbreeding and intraspecific competition (Danchin and Cam 2002, Coulson 2016, Acker et al. 2018) as well as the movement to a better-quality habitat with more resources (Spear et al. 2002, Antaky et al. 2021). However, moving to a new territory means a lack of familiarity with available resources and potential predators, which exposes individuals to threats such as failed reproduction or mortality (Renken and Smith 1995, Coulson 2016, Antaky et al. 2021).
Avian dispersal tendency is typically biased toward females (Greenwood and Harvey 1982, Clarke et al. 1997, Mabry et al. 2013), both in proportion and distance traveled (Clarke et al. 1997, Végvári et al. 2018). One of the most common hypotheses explaining primarily female biased dispersal is that males of many species are the more territorial sex and therefore more concerned with the defense and care of their territory so that they may attract mates (Greenwood 1980, Greenwood and Harvey 1982). However, the exact origins of sex-biased dispersal are still debated and there does not appear to be one hypothesis that sufficiently captures the explanation for the sex differences (Greenwood 1980, Clarke et al. 1997, Mabry et al. 2013). Also, the mechanisms that drive sex bias in natal dispersal might not necessarily match those that impact breeding dispersal, so natal and breeding dispersal should be analyzed separately (Végvári et al. 2018).
Historically, it was assumed that most seabirds exhibit a high level of philopatry, however, this assumption may be biased because it is easier to identify individuals that have stayed compared to those that moved (Coulson 2016). Larids (gulls, terns, and skimmers) are primarily monogamous, colonial, or semi-colonial seabirds that nest in coastal areas (Winkler et al. 2020). Only 16 of 97 larid species from Winkler et al. (2020) have publications involving natal dispersal, and only 6 have data regarding sex-biased natal dispersal. Female-biased natal dispersal has been found in the Black-legged Kittiwake, Rissa tridactyla (Coulson and Nève de Mévergnies 1992), the Western Gull, Larus occidentalis (Spear et al. 2002), the Herring Gull, Larus argentatus (Chabrzyk and Coulson 1976), and the Common Tern, Sterna hirundo (Becker et al. 2008).
Female-biased natal dispersal in larids may exist because males are more involved in the acquisition and maintenance of the nesting territory, although females explore other colonies in search of the best mate (Spear et al. 2002, Becker et al. 2008). Additionally, higher productivity has been found in female Western Gulls that disperse, so reproductive success could be another explanation for female-biased natal dispersal (Spear et al. 2002). Our objective is to examine sex-biased natal dispersal in Black Skimmers (Rynchops niger) within southwest Florida to identify if natal dispersal tendency and distance differed between the sexes.
Black Skimmers are colonial-nesting larids that primarily breed on open beaches along the Atlantic and Gulf coasts of the United States (Gochfeld et al. 2020). This species is sexually dimorphic with males weighing more and having longer tarsus, culmen, and wing chord lengths than females (Quinn 1990, Forys et al. 2022a). Skimmers are monogamous and both sexes share parental responsibilities when caring for chicks. Pairs establish small territories within the colony at the beginning of the breeding season. Although both sexes defend the territories from conspecifics, males are responsible for significantly more of the aggressive encounters than females (Burger 1981). Because of a significant decline in the number of skimmers along with current threats from habitat loss, and disturbance and predation, the species is listed as threatened within the state of Florida (FWC 2022). Getting information on the frequency and sex bias of dispersal in the Black Skimmer is vital data for models that will help manage this species.
METHODS
Study area
We studied skimmers that were hatched and banded on beaches in Pinellas County, Florida (27°54′00.0″ N, 82°44′00.0″ W) a peninsula in West Central Florida, bordered by the Gulf of Mexico and Tampa Bay (Fig. 1). Pinellas is a highly urbanized county, and Black Skimmer colonies are easily accessible because they are often on busy public beaches. Banded skimmers were resighted throughout Florida and surrounding states.
Most of the skimmer colonies occurred on barrier islands connected to the mainland via bridges. Three relatively large and accessible colonies occurred on these developed barrier islands: Clearwater Point, Indian Shores, and St. Pete Beach (Fig. 1). The colonies have shifted slightly among breeding seasons, but none moved more than 1.5 km. Clearwater Point is a private beach behind a condominium complex that experienced very few human-related disturbances (Forys et al. 2022b). Indian Shores is located on a more crowded beach next to condominiums and a volleyball court, which are frequently used by tourists. St. Pete Beach is a heavily trafficked public beach that borders hotels and condominiums. All three colonies were protected by bird stewards who educate the public and decrease human disturbance (Forys et al. 2022b). Numbers of pairs and nests fluctuated among colonies and years, but maximum nest count ranged from 85-255 (Table 1). Productivity was variable but was similar to other published studies of North American colonies (Gochfeld et al. 2020).
Data collection and analysis
During 2015 to 2019, a total of 225 3-4-week-old Black Skimmer chicks were caught using hand nets at the 3 Pinellas County natal colonies (Clearwater Point, Indian Shores, and St. Pete Beach) and banded with 12- or 20-mm field readable bands. Banded birds were resighted from 2015-2022 by members of the Florida shorebird alliance (FSA), a network of volunteers, biologists, and beach managers that is committed to advancing shorebird and seabird conservation in Florida (FWC 2022). Trained surveyors followed Florida’s breeding bird protocol at colonies at least once each week and recorded the number of nests, chicks, adults, and any banded birds and entered this data into the Florida shorebird database (FSD 2022). For example, in 2022, the 42 skimmer colonies in Florida were officially monitored 586 times (FWC 2022). Additional band resightings came from FSA monitoring outside the official surveys, members of the public who found our web and Facebook pages, as well as partners who monitor skimmers through the Gulf and Atlantic Coasts. Some colonies were surveyed for bands more than others, but all colonies were monitored several times each month.
The natal colony of each bird was recorded when it was banded as a flightless chick. We recorded the locations where each banded skimmer was seen breeding for the first time to compare to their natal colonies. Banded skimmers were only included in our data if they were observed to be mating or incubating. Black Skimmers do not reach sexual maturity until their second or third year (Gochfeld et al. 2020), so the earliest a skimmer could be observed and recorded to be breeding would be two years after hatching. Hence, even though the skimmers were banded during 2015-2019, our study represents data collected during the 2017-2022 breeding seasons (May 15-September 15).
In total, of the 225 flightless chicks we banded, we determined the first breeding location for only 86. Twenty individuals died before leaving the colony, primarily during a salmonellosis outbreak associated with the release of raw sewage in 2016 (Shender et al. 2022). Many banded birds were not seen after fledging, but the majority of the birds seen after their first year were seen frequently in subsequent years. Throughout their range, skimmer survival from the time of fledging to year one is believed to be low, although skimmer adult survival is generally very high (Gochfeld et al. 2020).
Profile photographs of the 86 Black Skimmers used in our analyses were taken once they were adults, and sex was determined using a verified method described by Forys et al. (2022a). Of the 86 skimmers, we identified 29 females and 57 males. The dispersal distances between the skimmers’ respective natal and breeding colonies were measured by drawing a straight line between the two colonies using ArcGIS Pro v.2.8.1 (ESRI 2021). Birds were considered philopatric if they nested at their natal colony (dispersal distance = 0 km). The three natal colonies (St. Pete Beach, Indian Shores, and Clearwater Point) were also considered dispersal colonies because some skimmers moved between these colonies. Statistical tests were done using R v.4.2.1 (R Core Team 2022). To determine if the proportion of female natal dispersers differed significantly from males, we conducted a chi-square test of independence. To test for a difference between the dispersal distances of the dispersers for each sex, we used a Wilcoxon rank sum test because our data were not normally distributed (p < 0.05).
RESULTS
About 72% of females dispersed compared to 61% of males (Fig. 2), and this difference was not significant (χ² = 0.60, P = 0.44). Natal dispersal distances ranged from 12.9 - 242.6 km (Fig. 2). On average, female natal dispersers traveled 43.8 km (median = 27.2 km, range = 15.3 - 217.9 km), while males traveled 51.4 km (median = 27.2 km, range = 12.9 - 242.6 km). Natal dispersal distance did not differ significantly between the dispersers of each sex, 35 males, and 21 females (W = 354, P = 0.59).
Of the 86 Black Skimmers that dispersed, 81% of females and 83% of males traveled ≤ 50 km from their natal colony (Fig. 2). The proportion of skimmers that dispersed was similar among each of the three natal colonies (61% from St. Pete Beach, 72% from Indian Shores, and 67% from Clearwater Point). See Tables 1-2.
DISCUSSION
We found that over half of all skimmers exhibited natal dispersal, and the proportion that dispersed was similar for males and females. In addition, we did not find a statistically significant difference between the natal dispersal distances for each sex. The majority of both males and females that dispersed moved to colonies within 50 km of their natal colony. A similar proportion (3% of females and 5% of males) dispersed > 200 km. This skew in dispersal distance where only a few individuals travel far from their natal colony has also been recorded in other avian species (Sutherland et al. 2000, Spear et al. 2002, Luna et al. 2020). Overall, our natal dispersal proportion and distance findings are compatible with those from Delgado et al. (2020), who found no sex bias for Yellow-legged Gulls in either natal dispersal proportion or distance traveled. Our findings support Coulson’s (2016) claims that philopatry and dispersal vary widely between bird species and that philopatric tendencies in seabirds may not be as high as what was previously thought due to limitations in recapturing birds that travel great distances (Koenig et al. 1996).
Our research has implications for the conservation and management of Black Skimmers. Seabirds, such as Black Skimmers, exist as a metapopulation of individual colonies that are connected in terms of demographic and genetics through dispersal (Oro 2003). Fortunately, finding that over half of the male (61%) and female (72%) Black Skimmers in this study dispersed is advantageous for their genetic diversity and the prevention of inbreeding. Declining species that experience habitat fragmentation and loss can be more susceptible to inbreeding and low genetic diversity, but a high level of dispersal can counteract this (Dayton and Szczys 2021, Rönkä et al. 2021). In addition, dispersing individuals can genetically and demographically “rescue” declining colonies by adding individuals and because both males and females disperse, colonies are less likely to have sex ratio imbalances that would decrease productivity for a monogamous species (Yannic et al. 2016).
The finding that most male and female natal dispersers in our study dispersed no more than 50 km from their natal colonies and most of them dispersed to the south is potentially important for future population viability models. Although southwest Florida supports the largest number of Black Skimmers in the state (Fig. 1), there are colonies in northern Florida, and 14 of the 86 Black Skimmers in our study have spent time outside of the breeding season near some of these colonies, but there is no record of them nesting. This low level of dispersal to the north might be important if skimmer populations continue to decline. This study shows the locations of where Black Skimmers in southwest Florida are dispersing and nesting, which should help guide and improve conservation and management efforts to protect this species.
Our study is the first that focuses on sex-biased natal dispersal within Black Skimmers, but it does have several limitations. All three natal colonies were from one region in southwest Florida and future research should include colonies throughout more of their range. We did not find evidence that the more human disturbed colonies (St. Pete Beach and Indian Shores) had a higher proportion of banded birds dispersing than the less disturbed Clearwater Point colony, but it would be interesting to conduct a more in-depth analysis of the roles that disturbance, predation, and colony reproductive success have on the proportion of males and females that disperse.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.
ACKNOWLEDGMENTS
We thank the Eckerd College Ford Scholar Program for funding this research, as well as the Audubon Florida Bird Steward Program and the Florida Shorebird Alliance for their commitment to collecting Black Skimmer data. In addition, this project would not have been possible without the data gathered by citizen science from these organizations. Black Skimmers were captured and marked under M. Korosy’s (23627), and E. Forys (24258) federal bird banding permits.
DATA AVAILABILITY
All the relevant data appear in Appendix 1.
LITERATURE CITED
Acker, P., C. Francesiaz, A. Béchet, N. Sadoul, C. M. Lessells, A. S. Pijl, and A. Besnard. 2018. Insights on dispersal and recruitment paradigms: sex-and age-dependent variations in a nomadic breeder. Oecologia 186:85-97. https://doi.org/10.1007/s00442-017-3972-7
Antaky, C. C., L. Young, J. Ringma, and M. R. Price. 2021. Dispersal under the seabird paradox: probability, life history, or spatial attributes? Marine Ornithology 49:1-8. http://www.marineornithology.org/PDF/49_1/49_1_1-8.pdf
Becker, P. H., T. H. G. Ezard, J. D. Ludwigs, H. Sauer‐Gürth, and M. Wink. 2008. Population sex ratio shift from fledging to recruitment: consequences for demography in a philopatric seabird. Oikos 117(1):60-68. https://doi.org/10.1111/j.2007.0030-1299.16287.x
Burger, J. 1981. Aggressive behaviour of Black Skimmers (Rynchops niger). Behaviour 76(3-4):207-222. https://doi.org/10.1163/156853981X00086
Chabrzyk, G., and J. C. Coulson. 1976. Survival and recruitment in the Herring Gull Larus argentatus. Journal of Animal Ecology 45(1):187-203. https://doi.org/10.2307/3774
Clarke, A. L., B. E. Sæther, and E. Røskaft. 1997. Sex biases in avian dispersal: a reappraisal. Oikos 79(3):429-438. https://doi.org/10.2307/3546885
Coulson, J. C. 2016. A review of philopatry in seabirds and comparisons with other waterbird species. Waterbirds 39(3):229-240. https://doi.org/10.1675/063.039.0302
Coulson, J. C., and G. Nève de Mévergnies. 1992. Where do young Kittiwakes Rissa tridactyla breed, philopatry or dispersal. Ardea 80(1):187-197.
Danchin, E., and E. Cam. 2002. Can non-breeding be a cost of breeding dispersal? Behavioral Ecology and Sociobiology 51:153-163. https://doi.org/10.1007/s00265-001-0423-5
Dayton, J., and P. Szczys. 2021. Metapopulation connectivity retains genetic diversity following a historical bottleneck in a federally endangered seabird. Ornithological Applications 123(4):1-17. https://doi.org/10.1093/ornithapp/duab037
Delgado, S., A. Aldalur, A. Herrero, and J. Arizaga. 2020. No evidence supporting sex-dependent differential movements and survival in Yellow-legged Gulls. Ardea 108(2):183-190. https://doi.org/10.5253/arde.v108i2.a4
Environmental Systems Research Institute (ESRI). 2021. ArcGIS Pro. Version 2.8.1. Environmental Systems Research Institute, Redlands, California, USA.
Florida Fish and Wildlife Conservation Commission (FWC). 2022. Florida Shorebird Alliance monitoring data at work. Monitoring data retrieved from the Florida shorebird database (FSD). Florida FWC Commission, Tallahassee, Florida, USA. https://flshorebirdalliance.org/media/1267/2021fsamonitoringdataatwork.pdf
Florida Shorebird Database (FSD). 2022. An online tool for entering and exploring data on Florida’s shorebirds and seabirds. Florida Fish and Wildlife Conservation Commission, Tallahassee, Florida, USA. www.FLShorebirdDatabase.org
Forys, E. A., C. Naundorff, K. M. Kennedy, and P. T. Paddock. 2022a. Use of morphometric measurements of photographs of a sexually dimorphic bird to determine sex. Waterbirds 44(3):324-329. https://doi.org/10.1675/063.044.0307
Forys, E. A., S. K. Beres, A. L. McCay, and O. N. Spicer. 2022b. Infanticide in highly urbanized colonies of Black Skimmers Rynchops niger. Marine Ornithology 50:43-47. http://www.marineornithology.org/article?rn=1458
Gochfeld, M., J. Burger, and K. L. Lefevre. 2020. Black Skimmer (Rynchops niger). Version 1.0. Birds of the world. Cornell Lab of Ornithology, Ithaca, New York, USA. https://doi.org/10.2173/bow.blkski.01
Greenwood, P. J. 1980. Mating systems, philopatry and dispersal in birds and mammals. Animal Behaviour 28(4):1140-1162. https://doi.org/10.1016/S0003-3472(80)80103-5
Greenwood, P. J., and P. H. Harvey. 1982. The natal and breeding dispersal of birds. Annual Review of Ecology and Systematics 13:1-21. https://doi.org/10.1146/annurev.es.13.110182.000245
Koenig, W. D., D. Van Vuren, and P. N. Hooge. 1996. Detectability, philopatry, and the distribution of dispersal distances in vertebrates. Trends in Ecology and Evolution 11(12):514-517. https://doi.org/10.1016/S0169-5347(96)20074-6
Luna, Á., A. Palma, A. Sanz-Aguilar, J. L. Tella, and M. Carrete. 2020. Sex, personality and conspecific density influence natal dispersal with lifetime fitness consequences in urban and rural Burrowing Owls. PLoS ONE 15(2):e0226089. https://doi.org/10.1371/journal.pone.0226089
Mabry, K. E., E. L. Shelley, K. E. Davis, D. T. Blumstein, and D. H. Van Vuren. 2013. Social mating system and sex-biased dispersal in mammals and birds: a phylogenetic analysis. PloS ONE 8(3):e57980. https://doi.org/10.1371/journal.pone.0057980
Oro, D. 2003. Managing seabird metapopulations in the Mediterranean: constraints and challenges. Scientia Marina 67(Suppl.2):13-22. https://doi.org/10.3989/scimar.2003.67s213
Quinn, J. S. 1990. Sexual size dimorphism and parental care patterns in a monomorphic and a dimorphic larid. Auk 107(2):260-274. https://doi.org/10.2307/4087608
R Core Team. 2022. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Renken, R. B., and J. W. Smith. 1995. Interior Least Tern site fidelity and dispersal. Colonial Waterbirds 18(2):193-198. https://doi.org/10.2307/1521480
Rönkä, N., V. M. Pakanen, A. Pauliny, R. L. Thomson, K. Nuotio, H. Pehlak, O. Thorup, P. Lehikoinen, A. Rönkä, D. Blomqvist, K. Koivula, and L. Kvist. 2021. Genetic differentiation in an endangered and strongly philopatric, migrant shorebird. BMC Ecology and Evolution 21(125):1-12. https://doi.org/10.1186/s12862-021-01855-0
Shender, L. A., T. Cody, M. Ruder, H. Fenton, K. D. Niedringhaus, J. Blanton, and E. A. Forys. 2022. Heavy rainfall, sewer overflows, and salmonellosis in Black Skimmers (Rynchops niger). EcoHealth 19:203-215. https://pubmed.ncbi.nlm.nih.gov/35655049/
Spear, L. B., P. Pyle, and N. Nur. 2002. Natal dispersal in the Western Gull: proximal factors and fitness consequences. Journal of Animal Ecology 67(2):165-179. https://doi.org/10.1046/j.1365-2656.1998.00190.x
Sutherland, G. D., A. S. Harestad, K. Price, and K. P. Lertzman. 2000. Scaling of natal dispersal distances in terrestrial birds and mammals. Conservation Ecology 4(1):16. https://doi.org/10.5751/ES-00184-040116
Végvári, Z., G. Katona, B. Vági, R. P. Freckleton, J. M. Gaillard, T. Székely, and A. Liker. 2018. Sex‐biased breeding dispersal is predicted by social environment in birds. Ecology and Evolution 8(13):6483-6491. https://doi.org/10.1002/ece3.4095
Winkler, D. W., S. M. Billerman, and I. J. Lovette. 2020. Gulls, terns, and skimmers (Laridae). Version 1.0. In S. M. Billerman, B. K. Keeney, P. G. Rodewald, and T. S. Schulenberg, editors. Birds of the world. Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.larida1.01
Yannic, G., J. M. Yearsley, R. Sermier, C. Dufresnes, O. Gilg, A. Aebischer, M. V. Gavrilo, H. Strøm, M. L. Mallory, R. I. Guy Morrison, H. Grant Gilchrist, and T. Broquet. 2016. High connectivity in a long-lived high-Arctic seabird, the Ivory Gull Pagophila eburnea. Polar Biology 39:221-236. https://doi.org/10.1007/s00300-015-1775-z
Fig. 1
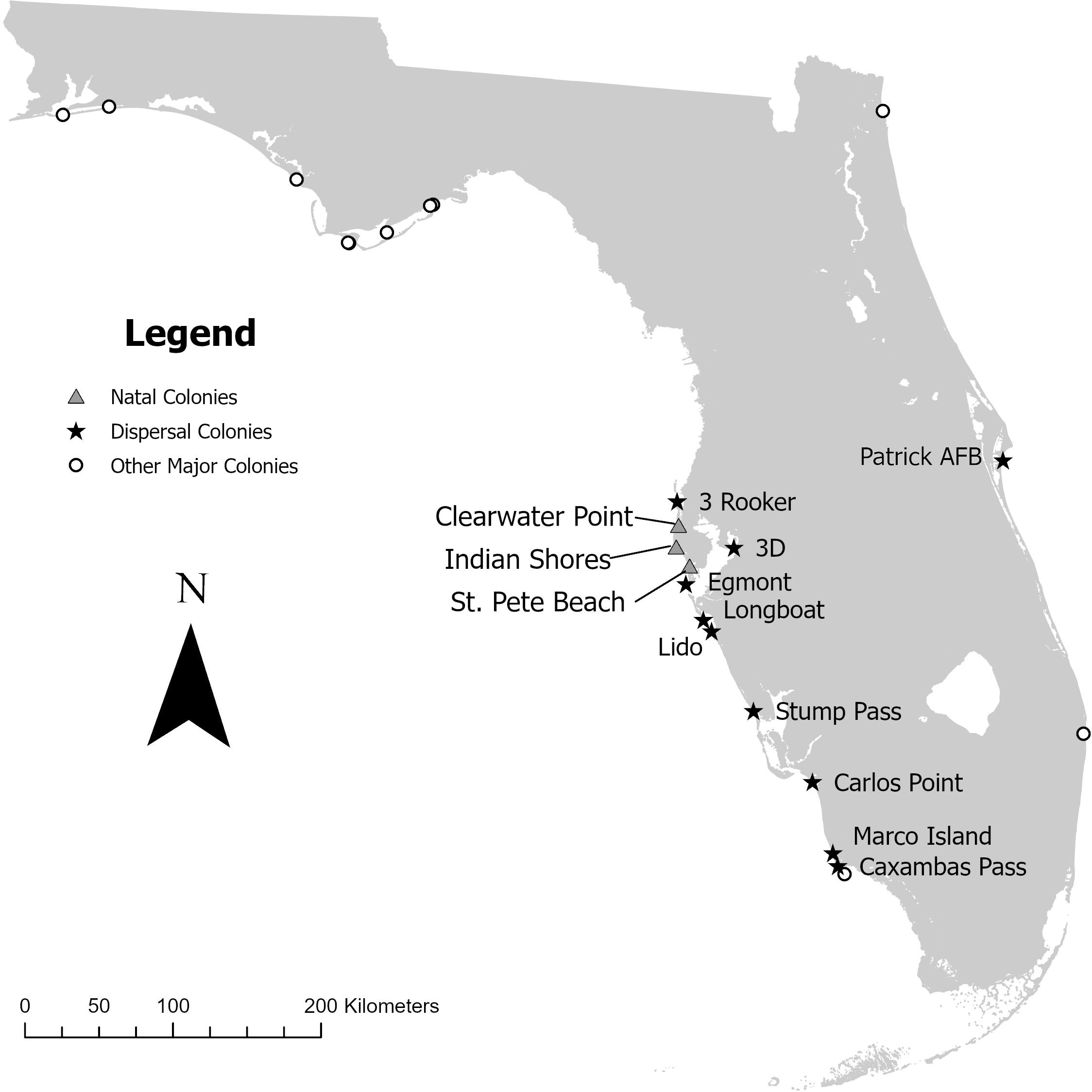
Fig. 1. Study area map displaying the three Black Skimmer (Rynchops niger) natal colonies of the individuals used in the study and the southwest Florida skimmer colonies to which they dispersed. All three natal colonies were also considered dispersal colonies, as some of the skimmers moved between those natal colonies.

Fig. 2
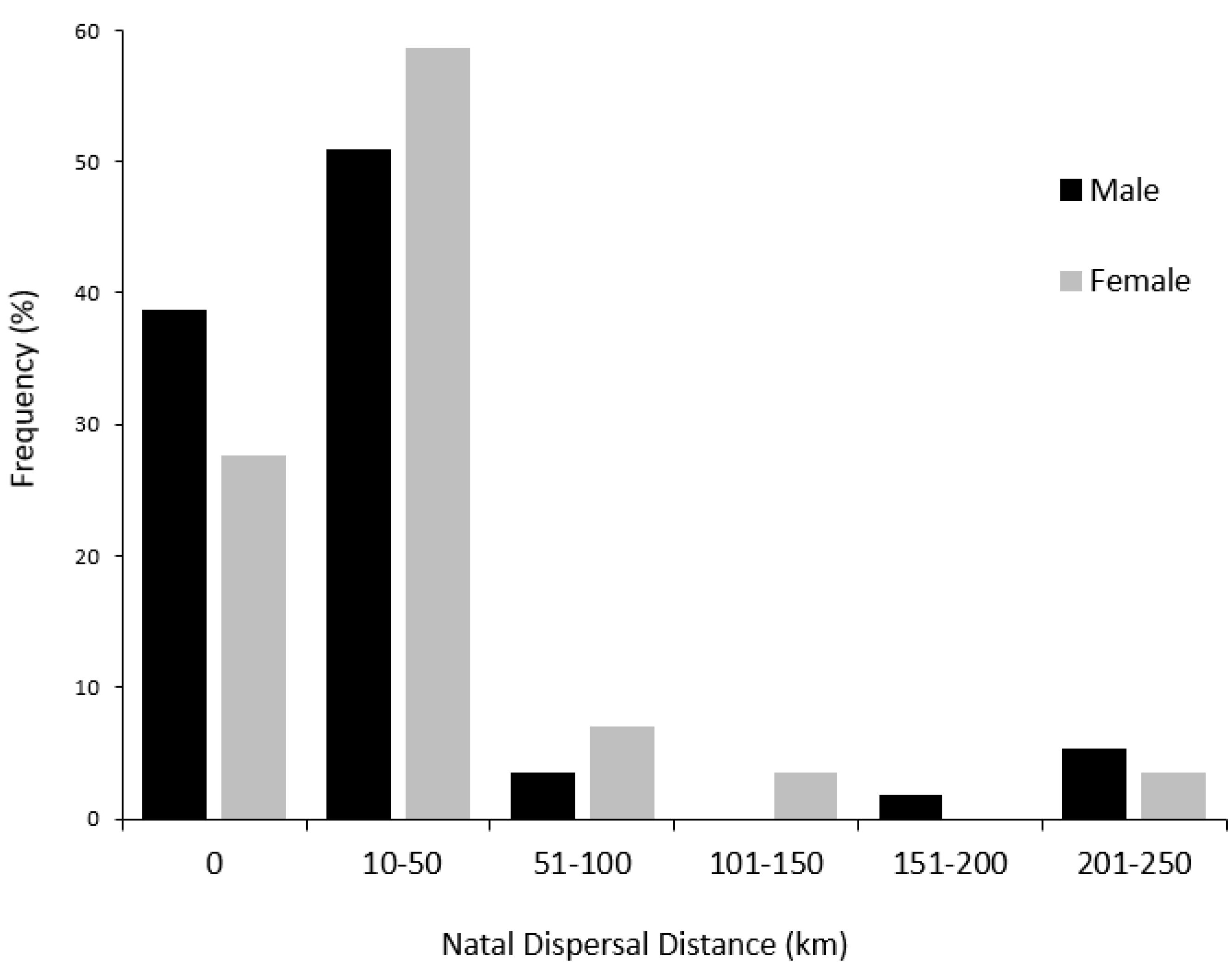
Fig. 2. Percentages of male (n = 57) and female (n = 29) Black Skimmers (Rynchops niger) with their respective distances they traveled from their natal colony to their dispersal colony (km).

Table 1
Table 1. Productivity data for the three natal Black Skimmer (Rynchops niger) colonies in Pinellas County, Florida from 2015-2019, including the number of banded birds that were philopatric and the number that dispersed to breed at a new colony.
| Year | Natal Colony | Max Nests (n) | Max Fledglings (n) | Productivity (fledgling/nest) |
# Philopatric | # Dispersed | |||
| 2015 | Clearwater Point | 150 | 125 | 0.83 | 0 | 0 | |||
| Indian Shores | 250 | 55 | 0.22 | 2 | 3 | ||||
| St. Pete Beach | 148 | 169 | 1.14 | 6 | 8 | ||||
| 2016 | Clearwater Point | 254 | 146 | 0.57 | 0 | 0 | |||
| Indian Shores | 90 | 33 | 0.37 | 0 | 2 | ||||
| St. Pete Beach | 92 | 103 | 1.12 | 3 | 7 | ||||
| 2017 | Clearwater Point | 180 | 177 | 0.98 | 3 | 5 | |||
| Indian Shores | 85 | 169 | 1.99 | 1 | 4 | ||||
| St. Pete Beach | 135 | 236 | 1.75 | 5 | 6 | ||||
| 2018 | Clearwater Point | 65 | 41 | 0.63 | 0 | 0 | |||
| Indian Shores | 70 | 34 | 0.49 | 3 | 3 | ||||
| St. Pete Beach | 230 | 149 | 0.65 | 4 | 2 | ||||
| 2019 | Clearwater Point | 128 | 91 | 0.71 | 1 | 3 | |||
| Indian Shores | 152 | 85 | 0.56 | 1 | 6 | ||||
| St. Pete Beach | 250 | 227 | 0.91 | 1 | 7 | ||||
Table 2
Table 2. Count of banded Black Skimmers (Rynchops niger) that either nested at their natal colony where they were banded, or moved from one of the three natal colonies (St. Pete Beach, Indian Shores, and Clearwater Point) to one of the dispersal colonies where they bred for the first time.
| Dispersal Colony | Natal Colony | ||
| St. Pete Beach | Indian Shores | Clearwater Point | |
| St. Pete Beach | 19 | 11 | 0 |
| Indian Shores | 6 | 7 | 5 |
| Clearwater Point | 4 | 1 | 4 |
| 3D | 1 | 1 | 0 |
| 3 Rooker | 0 | 1 | 1 |
| Carlos Point | 1 | 1 | 0 |
| Caxambas Pass | 0 | 1 | 0 |
| Egmont Key | 2 | 0 | 0 |
| Lido Key | 13 | 2 | 1 |
| Longboat Key | 0 | 0 | 1 |
| Marco Island | 1 | 0 | 0 |
| Patrick Airforce Base (rooftop) | 1 | 0 | 0 |
| Stump Pass | 1 | 0 | 0 |
| Total | 49 | 25 | 12 |








