The following is the established format for referencing this article:
Guppy, M., S. Guppy, P. Withers, and R. Marchant. 2023. Home range sizes of 11 bird species on a 10-ha forest site in southeast Australia. Journal of Field Ornithology 94(1):8.ABSTRACT
We used a simple and objective method of determining home range size for individuals of a bird community in southeast Australia. The community consisted of 11 species, which represented nine genera, five families, and a range of diets and nesting behaviors. The vegetation on the 10-ha site comprises a mixture of eucalypt forest; dense thickets of cycads, casuarinas, and ti-tree; a bushy and grassy powerline clearing 30-m wide running the length of the site; 1 ha of dense swamp paperbark trees; several small dams containing various reeds; and a riparian environment of a variety of different shrubs. Data were collected from 490 color-banded individuals of both sexes over seven to eight breeding seasons, and between 40 and 966 sightings were recorded per species. Species was a significant predictor of home range size (26% of the variance), but this was mainly because three species had large home ranges compared to the remaining species, which had similar but variable home ranges. Breeding season (as indicated by year), sex, and number of pairs were also significant predictor factors but together accounted for only 4% of the variance. The Southern Oscillation Index (a measure of the El Niño-Southern Oscillation, a major climate factor that is related to rainfall in eastern Australia) was not a significant predictor. The high residual variation (70%) indicated that each species had inherently variable home range sizes. Home range sizes were generally dissimilar (both lower and higher) to those of the same species in the literature but are consistent year to year at our study site. We suggest that wide variation in home range sizes of species is the result of both between-site habitat variation and within-site microhabitat variation, and is therefore not unexpected.
RESUMEN
e utilizó un método sencillo y objetivo para determinar el tamaño de la zona de residencia de los individuos de una comunidad de aves del sureste de Australia. La comunidad estaba formada por 11 especies, que representaban a nueve géneros, cinco familias y una amplia gama de dietas y comportamientos de nidificación. La vegetación del lugar, de 10 ha, comprende una mezcla de bosque de eucaliptos; densos matorrales de cícadas, casuarinas y cordyline; un claro de tendido eléctrico arbustivo y herbáceo de 30 m de ancho a lo largo del lugar; 1 ha de densos árboles de corteza de papel de pantano; varias pequeñas represas que contienen diversos juncos; y un entorno ribereño de una variedad de arbustos diferentes. Se recogieron datos de 490 individuos de ambos sexos marcados con bandas de color durante siete u ocho temporadas de cría, y se registraron entre 40 y 966 avistamientos por especie. La especie fue un predictor significativo del tamaño de la zona de residencia (26% de la varianza), pero esto se debió principalmente a que tres especies tenían grandes zonas de residencia en comparación con las especies restantes, que tenían zonas de residencia similares pero variables. La época de cría (indicada por el año), el sexo y el número de parejas también fueron factores de predicción significativos, pero juntos sólo representaron el 4% de la varianza. El Índice de Oscilación del Sur (una medida de la Oscilación del Sur-El Niño, un importante factor climático relacionado con las precipitaciones en el este de Australia) no fue un factor predictivo significativo. La elevada variación residual (70%) indica que el tamaño de las zonas de residencia de cada especie es intrínsecamente variable. En general, los tamaños de las zonas de residencia eran distintos (tanto inferiores como superiores) a los de las mismas especies en la bibliografía, pero son constantes de un año a otro en nuestro lugar de estudio. Sugerimos que las amplias variaciones en los tamaños de las zonas de residencia de las especies son el resultado tanto de la variación del hábitat entre sitios como de la variación del microhábitat dentro del sitio, y por lo tanto no es inesperado.
INTRODUCTION
The significance of territories in the natural history of birds (Howard 1920) quickly progressed to a worldwide interest in the function of these territories and to a consideration of the distinction between a territory (defended) and a home range (not defended), which is frequently not obvious or considered (Birkhead 2008, Anich et al. 2009, Lack 2015). There are many possible functions of both territoriality and a defined home range, which relate, for example, to pair formation, food supply, nesting sites, predation, and population density. The search for evidence of these putative connections, as well as efforts to quantify their significance and describe how their interactions determine territory or home range sizes and shapes have been in progress for at least 80 years (e.g., Noble 1939, Seastedt and Maclean 1979, Schieck and Hannon 1993, Adams 2001, Marshall and Cooper 2004, Yoon 2014). In Australia, various space-related questions have been investigated (since at least 1950) for a variety of species and habitats (Erickson 1950, Bell and Ford 1987, Marchant 1987, Ambrose and Davies 1989, Tidemann 1990, Bridges 1994, Green and Cockburn 2001, Chan and Augusteyn 2003, Clarke et al. 2003, Van Dongen and Yocom 2005, Debus 2006, Colombelli-Négrel 2016).
What is obvious from the plethora of literature on this subject is first, that any factor associated with either a territory or a home range is habitat- (or study site-) and species-dependent, and second, that the interactions among these factors, and associated bird behaviors, are many, complex, and still being discovered and defined. These two statements certainly apply to an obvious and fundamental aspect of territories/home ranges: their size. A summary of what was known about territory size in 2001 (Adams 2001) stated that most studies show little or no effect of food supply on territory size; that the rate of weight gain by an individual is not necessarily linked to territory size; that the size of territories is linked to various combinations of crowding, and body and group sizes; and that relative (to neighbors’) body sizes and ages can be a better predictor of territory size than actual body sizes and ages. Since that review, techniques have changed, and many more species and habitat types have been investigated. However, it is difficult to find common principles because much of the data concerning territory size are influenced by habitat, species, and the study site. The results of these more recent studies variously show that territory size can be related to body size (Ottaviani et al. 2006), or not (Mathias and Duca 2016, Chaves et al. 2019), to group size (Duca and Marini 2014), and to species (Mathias and Duca 2016). It can decrease with increasing food abundance (Atuo and Manu 2013, Haché et al. 2013), can be related to foliage density and forest heterogeneity (Marshall and Cooper 2004, Vargas et al. 2011, Skorupski et al. 2018) and the number of neighboring territories (Chaves et al. 2019), and can increase with urbanization (Juárez et al. 2020).
Isolating the effects of the numerous factors on territory size is difficult, so there are few studies that attempt to separate out, for example, the effect of species on territory size. There are studies that have involved 13–17 species in the same habitat or on the same site (Haila et al. 1996, Stouffer 2007, Holmes 2011), but most of these studies do not specifically investigate the relation between home range size and species. In Australia, there are only two such studies, both of which used color-banded individuals and targeted three species of Fairy-wren which are physically and to a large extent behaviorally similar (Tidemann 1990, Chan and Augusteyn 2003). There were differences in home range sizes among the three species, but they were not consistent between seasons. These studies are further complicated by the fact that the sizes of home ranges for a particular species can vary by up to 20-fold among studies or habitats; e.g., for the Superb Fairy-wren (Malurus cyaneus) (Tidemann 1990, Chan and Augusteyn 2003, Skorupski et al. 2018).
Additional difficulties arise when one considers how the sizes of home ranges are determined (from now on, we refer only to home ranges, which we define simply as where the bird is seen). Most studies have used, and are still using, either planar areas based on a spatial map and recognition of individual birds, or bird densities gleaned from either observations or a variety of data sources (Seasteadt and MacLean 1979, Ambrose and Davies 1989, Bridges 1994, Clarke et al. 2003, Marshall and Cooper 2004, Debus 2006, Stouffer 2007, Duca and Marini 2014, Yoon 2014). The collection of the data for these methods, namely the sightings of individuals and the recording of bird densities, is notoriously observer- and effort-dependent (Anich et al. 2009).
A strategy for overcoming these problems would be to compare the home range sizes of different species in the same habitat, using data derived from many and unambiguous sightings of multiple individuals from each species. Such a strategy would at least provide insights into the question of whether there are inherent sizes of the home ranges associated with different species. The study we present here for a community of both permanent residents and seasonal visitors on a forest site in southeast Australia was part of a broader project during which a range of data were collected for 44 species that bred on the site over 8 years. The number of breeding pairs each season ranged from 56 to 109, and the number of nests active on any one day reached a maximum of 35. The average number of pairs per season of the 11 species in the current home range study varied from 1 (White-throated Treecreeper [Cormobates leucophaeus]) to 15 (Yellow-faced Honeyeater [Lichenostomus chrysops]) (Guppy et al. 2021). The study of home ranges described here used a specific subset of the data collected during the broader project, which comprised large numbers of unambiguous sighting data for color-banded and breeding individuals of both sexes from 11 species in the same habitat over 7–8 breeding seasons (depending on the species). We developed a novel method for quantifying relative and actual home range sizes that allows rapid, objective, and simple calculation and analysis of mean home range sizes for both males and females of the 11 species. We investigated the influence of five factors on home range size:
1. and 2. Species and group size/density/crowding have been shown to affect home range size (see Introduction). Therefore, we investigated the effect of species and the numbers of breeding pairs of each species.
3. There is one study on the territories of the males and females of pairs of the Tropical Oriole (Icterus ictertus)(Odum et al. 2019), which showed male and female territory size, shape, and location to be similar. However, we reasoned that the effect of sex should nevertheless be investigated due to the differences in territorial behavior between the males and females of many species.
4. The effect of year will identify any long-term trends, such as climate change, but would also highlight particular years in which factors such as the El Niño-Southern Oscillation were particularly favorable or unfavorable.
5. The El Niño-Southern Oscillation is a major climatic factor in eastern Australia, which is quantified by the Southern Oscillation Index (SOI). When the index is positive, more rain occurs; when it is negative, there is less rain (Nicholls 1991). We have already shown that our bird community responds to a positive index with an increase in the number of breeding pairs and earlier egg laying (Marchant et al. 2016, 2021). Thus, it seemed possible that SOI might also influence the size of home ranges.
METHODS
Study site
The study site (35°52’ S, 150°03’ E) was a 10-ha forest area (approximately 200 x 500 m; 100 m above sea level), 8 km inland from the southeast coast, about 300 km south of Sydney, Australia, near the town of Moruya (to see the site on Google Earth, go to 1708 Maulbrooks Road Mogendoura). The site is on the west side of a ridge (Fig. 1[A]) and has a mostly gentle sloping aspect to an intermittent small creek in the west (Fig. 1[B]) but rises steeply near the eastern ridgetop (Fig. 1[C]). Vegetation comprises a mixture of forest (blackbutt [Eucalyptus pilularis], stringybarks [E. globoides and E. muellerana], spotted gum [C. maculata], and lesser content of grey ironbark [E. paniculata] and rough-barked apple [Angophora floribunda], reaching heights of 30 m), thickets of burrawang or cycad (Macrozamia communisa) (Fig. 1[D]), several areas of black she-oak (Allocasuarina littoralis [10 m]) and tick bush (Kunzea ambigua [4 m]), a powerline clearing 30-m wide running the length of the site comprising tick bush, bracken (Pteridium esculentum), and open grassland (Fig. 1[E]), and 1 ha of dense swamp paperbark (Melaleuca ericifolia [5–10 m]) (Fig. 1[F]). In addition, there are several small dams containing cumbungi (Typha orientalis), spikerush (Eleocharis sp.), and reed (Phragmites australis) (Fig. 1[G]), and a riparian environment of a variety of different shrubs (water gum [Tristania laurina], grey myrtle [Backhousia myrifolia], [Melaleuca spp.], [Callistemon spp.]), and the river oak (Casuarina cunninghamiana) next to a rocky stream bed (Fig. 1[B]). Similar habitat is widespread for at least 5 km inland of a 150-km stretch of coast between Ulladulla and Bermagui, New South Wales (NSW) (Austin 1978). The site adjoins State Forest and is situated in a mixed landscape of forest and cleared grazing land, with forest as the dominant component. Aerial photographs of the nearby State Forest (Forestry Corporation of NSW, Southern Region, personal communication) show that few and only small changes to the area of forested land have occurred since 1949.
Fieldwork
The fieldwork is described in detail in Guppy et al. (2021). The site was divided into 50- x 50-m squares by tracks running north–south and east–west. To identify breeding pairs of each species, all nests were found, individual birds were linked with each nest, and nests were monitored until the young birds fledged or the nest failed (usually by predation). Color-banding was used to identify individuals. Birds were banded either at the nest or by systematically netting the entire site. Observations were made by two people (MG and SG) walking the grid, 25 m apart, on most (80–90%) days during the breeding season (August–January inclusive for the seasons 2007/2008–2014/2015), for a daily average period of 2.9 h. Walks covered 1.5–2.5 km, and the entire grid was completed every 3–4 days; therefore, each home range was monitored at least 45 times per season. Each time a banded bird was sighted, its position was recorded to the nearest intersection (on the 50- x 50-m grid). Recording to a finer accuracy was not realistic because the birds were often very mobile over the sighting period. Duplicate sightings (sightings at the same grid reference) were not recorded within each month, but recordings for each individual started anew each month.
Data
Data were collected for 490 individuals, both males and females, of 11 species (Table 1), representing nine genera and five families, and a range of different diets and nesting behaviors (Higgins et al. 2001, Higgins and Peter 2002). Data were analyzed only from birds (a) for which there were at least four sightings for that season, (b) that were color-banded, (c) whose sex was known, and (d) that were associated with a nest that progressed to having at least one egg. Table 1 shows the number of individuals of each species from which the data were derived, the average number of sightings for an individual of each species for one season, and the total number of sightings for each species. There were between 40 and 966 sightings per species over the 8 years for both males and females. The data are not from all individuals that were breeding each season because there were not enough sightings for some banded individuals.
The raw grid co-ordinates (X and Y) of the sightings were entered into an Excel file that comprised one individual for one season (one individual could provide data for several seasons, and would therefore be represented by more than one file). The sightings were converted into an XY position in metres on the grid, and then a home range centroid of all the sightings (the average X and average Y) for that individual for that season was calculated (e.g., Fig. 2). The Excel spreadsheet was then used to calculate the distance from the centroid of each sighting, and the average distance of all sightings from the centroid (ADC) for that individual for that season. The effect of the grid size on ADC was tested using models based on either a 50-m or a 20-m grid; the resulting ADCs differed by only 9%. Eighty percent of ADC values were based on more than six sightings (the maximum for an individual was 51). The average of all ADCs, for all individuals of each species, is termed the average ADC (male or female).
Relationship between average distance of all sightings from the centroids and home range areas
In order to convert ADCs into areas, the raw ADCs for selected individuals were used as the radius of a circle, and an area (m2) was calculated. This area is from now on referred to as an ADCArea. To test whether these areas reflected measured areas, the sightings for these selected individuals were plotted, shapes were determined by joining the outermost points, and areas (Measured Area [MA]; m2) were determined by dividing the home range into squares and right-angled triangles. Only the data for males were used for this analysis, and only the data from the first season were used for individuals that were on the site for more than one season (n = 113). In our case, all sightings were used to determine the Measured Area, in contrast to the usual approach used for the minimum convex polygon method (smallest polygon in which no internal angle exceeds 180 degrees [e.g., Marshall and Cooper 2004]). This was done for between two (White-throated Treecreeper) and 30 (Superb Fairy-wren) individuals of each species. There was a significant correlation between Measured Area and ADCArea (r2 = 0.78, P < 0.001); the regression (ADCArea = 0.91 MA + 0.16) had a slope close to 1 and an intercept near 0. Thus, the relationship was almost 1 to 1. ADCAreas were used for all analyses.
There are several advantages to using ADCs rather than Measured Areas. The data processing is simple and rapid because raw ADC values can be used for same-site comparisons, or they can be simply converted to an ADCArea for between-site comparisons. The data are objective; i.e., no choices are required about which points to include, and ADCs include the effect of repeat sightings (a co-ordinate where the bird is seen more than once), which is not the case for Measured Areas because repeated points sit on top of each other on the plots and are not specially weighted.
Data analysis
Probability plots showed ADCAreas were not normally distributed. A log10 transformation normalized the data, and all analyses were carried out on log10ADCArea. We used linear mixed models to determine which independent variables were related to log10ADCArea (the dependent variable). Our aim was to identify the relative influence of these variables rather than attempt to find which variables gave the best fit. Species and sex were fitted as fixed factors, while year and number of breeding pairs of each species for each year were included as fixed covariates. Year enabled any linear (or chronological) effects of time to be determined. Year identity (as year ID, a categorical variable) was entered as a random factor to allow for any non-independence of the data in a given year. The number of breeding pairs (pairs) showed no temporal autocorrelation over the seven breeding seasons (r = 0.13–0.40, all non-significant). Two interactions were also tested: species and sex, and species and number of pairs. Neither of these was significant (P = 0.51–0.73), and interactions were not included in the models.
Average SOI for April–July (AJSOI) (Bureau of Meteorology 2018), the months immediately before the start of breeding in August, was also included as a fixed covariate. AJSOI has been shown to influence the change in number of pairs between breeding seasons and the date of laying of the first egg for species on the site (Marchant et al. 2016, 2021). It seemed plausible that it could also affect the sizes of home ranges.
We used a method for obtaining variance explained (R2) from linear mixed models (Nakagawa and Schielzeth 2013) to determine the influence of the fixed factors and covariates. All analysis was carried out with SYSTAT, version 13.0 (Systat Software Inc., San Jose, CA, USA).
RESULTS
ADCArea varied among species (2.3-fold) (Table 2, Fig. 3), but most of this variation was due to the larger areas for the Variegated Fairy-wren (Malurus lamberti), White-throated Treecreeper, and Lewin’s Honeyeater (Meliphaga lewinii). The linear mixed models indicated that species was a significant factor, accounting for 26% of the variance in log10ADCArea (Table 3). Paired comparisons showed no significant differences among these three species, but the areas for the first two were significantly larger than those for the other eight species. Lewin’s Honeyeater was intermediate, with an area not significantly different from that of the Variegated Fairy-wren and White-throated Treecreeper but also not different from five of the remaining eight species. Each of these eight species showed some differences with several other species in this group, but because these interspecific differences did not usually exceed 0.5 ha, we attributed no meaning to them.
Year and number of pairs were also significant covariates, but year ID (a random and categorical factor) was not significant. The amount of variance accounted for by year and number of pairs was 1–2% each (as judged by the differences in R2 among models; see Table 3). There was a small decrease in ADCArea with time, but because the data spanned only 8 years, it would not be sensible to consider year a robust predictor of ADCArea. There was some relation between ADCArea and number of pairs: species with very few pairs (< 4) had somewhat larger areas (Fig. 4). Fourteen individuals had areas greater than 3 ha, but nine of them were Variegated Fairy-wrens, which suggests the effect was species specific rather than related to breeding density. Sex accounted for an additional 1%, although it was only marginally significant. The maximum difference between ADCAreas for males and females of a given species was 1.2-fold. AJSOI was not a significant covariate and added nothing to the explained variance. Together, year, number of pairs, and sex accounted for 4% of the variance. Clearly, species was the dominant factor.
The Akaike information criterion (AIC) scores suggest that model 3 (Table 3) provided the best description of the data, but model 1 was within 2 AIC units, and thus, was probably indistinguishable. Models 2 and 4 were less suitable, and model 5 was clearly not a good candidate because it was much more than 2 AIC units from model 3. The sizes of the estimated effects confirmed the dominance of species as a factor. The highest effect sizes for individual species ranged from 0.18 to 0.50 and were an order of magnitude higher than those for year or pairs (data not shown in Table 3).
Most of the variance (70–71%) in these models was contained in the error term. This is consistent with the large range of ADCAreas for each species (Fig. 3) and reflects variation among individuals; e.g., behavioral differences, and variation of an individual’s ADCArea over time (see Discussion).
DISCUSSION
Home ranges, as measured by ADCAreas, were very variable. Some of this was due to variation among species, but the majority was unaccounted for. Thus, each species had inherently variable home range sizes, and the other predictors of home range that we examined had little influence.
The two factors that we considered likely to be associated with the size of home ranges were sex and species. Sex was a marginally significant predictor factor (P = 0.06) and accounted for only 1% of the variance (Table 3). Even if the variation in the data was masking significant differences in the average ADC values between the sexes, any differences would be small (maximally 1.2-fold) (Table 2). This result suggests that, for these 11 species, although males may more obviously (through song and display) patrol home ranges (Langmore 2000, Fedy and Stuchbury 2005), the females use a similar-sized area. This is consistent with the data reported by Odum et al. (2019) on the Tropical Oriole (see Introduction). And although the male may be more obvious to other individuals of the same species, for most of the species, the human observers in this study sighted particular females as frequently as particular males (Table 1, column MS).
Species was a significant predictor factor and accounted for 26% of the variance. But apart from three species (Variegated Fairy-wren, White-throated Treecreeper, and Lewin’s Honeyeater) which had relatively large home range areas, the remaining species showed large overlaps (Fig. 3) due to the large variation in home range sizes for each species. This is despite these eight species representing a range of genera, families, weights, feeding strategies, diets, and nesting behaviors. Weight (Table 1) was not obviously related to territory size because the two species with the largest territories (White-throated Treecreeper [16–24 g] and Variegated Fairy-wren [6–11 g]) were at opposite ends of the weight spectrum. Weight was only weakly correlated with log10ADCArea (r = 0.16, P = 0.001).
With regard to feeding strategies, diets, and nesting behaviors, no quantitative data from this site that could be used to test the relation of these factors to home range size are available. The difficulty with conceiving a mechanism that explains the differences in the sizes of home ranges is strikingly demonstrated by the two Malurus wren species. These birds are in the same genus, are similar in size, breed in groups rather than in simple pairs, hunt in similar ways in similar habitats, eat similar prey, and build similar-looking nests in similar places, yet, their average home range sizes differ by a factor of 3.8. Home range sizes are the result of complex, interactive, and unknown factors.
In addition, different mechanisms could be at play for different species in relation to how each species interacts with the site. Consequently, concepts of home ranges, which assume reasonably small ranges in home range sizes for a species in a particular habitat and which are based on all species interacting with the site in the same way, may not explain the data. For example, the mean ADCArea for the White-throated Treecreeper may in fact be an underestimate. The males of this species had large home ranges but the lowest spread of values (Table 2). There were few pairs of this species on the site, and sightings ranged from the southern to the northern boundary of approximately one-third of the site. These characteristics are probably a consequence of a combination of the number of tree holes available for nesting (which are concentrated in one part of the site; note the consistency of the territory centroids for this species in the last column of Table 4) and the size of the site. Many of the sightings were on particular edges of the site and would not have been representative of the outer boundaries of the home range. A study on a larger site could uncover greater inherent variations in home ranges for this species.
Time and numbers of pairs were also significant negative factors (Table 3). According to the closest official meteorological site (9 km from the study site), average monthly temperatures have increased over the past 40 years by approximately 1°C (Bureau of Meteorology 2018). However, the variance accounted for by time was trivial (1%) (Table 3), which suggests that any effect related to time, such as local warming, had little effect on ADCArea. There was no other obvious but unmeasured physical factor that changed with time. Similarly, the amount of variance accounted for by the number of pairs of individual species each season was small (Table 3, Fig. 4), which suggests that home range size was not affected by density, except when densities were low. Even at low breeding densities, home range sizes exceeded those measured at higher densities only on certain occasions, and these low densities were confined largely to a single species (Variegated Fairy-wren). Although AJSOI is a major factor in determining how many pairs breed on the site (Marchant et al. 2016) and when they breed (Marchant et al. 2021), it was not a predictor of home range size (Table 3).
Clearly, much of the variation in the sizes of the home ranges was not related to any of the predictors examined so far. But home range sizes of each species could vary according to the positions of the home ranges on the site, which comprise different microhabitats. To this end, we constructed contour plots of centroid position and associated home range size for the seven species that nested over the entire site (Superb Fairy-wren, Variegated Fairy-wren, White-browed Scrubwren [Sericornis frontalis], Brown Thornbill [Acanthiza pusilla], Yellow-faced Honeyeater, Eastern Yellow Robin [Eopsaltria australis], and Rufous Whistler [Pachycephala rufiventris]) to look for common areas that were characterized by relatively small or large home ranges (see Fig. 5 for an example). We found only one area (approximately 2000 m2) that showed some commonality among species; i.e., five species had relatively large territories in this area. MG and SG know the site intimately, and it is not obvious to them why this area would require large territories. In general, we could find no evidence that home range sizes vary consistently with the location of the home range centroid.
The variation could also be a result of the same individual showing significant variations in home range sizes over different seasons, but with similar centroids; i.e., these individuals had their territories in the same area, but the size changed from year to year. This was indeed the case for some individuals, as shown by representative examples of the Superb Fairy-wren, Brown Thornbill, and Yellow-faced Honeyeater (Table 4). Home range sizes varied by at least 1.7-fold (and up to 2.3-fold) for the same individual between seasons, yet the centroids of the home ranges were similar across the seasons. These sizes were not consistently linked with the year for any of the three species (data not shown).
Some of the variation could also be a result of using the ADC method. There is one subjective decision that occasionally arises with this method, which could contribute to the residual variance. Our sightings were recorded as the co-ordinates of the nearest intersection of paths on the grid. In the worst-case scenario, a bird sighted in the middle of a 50- x 50-m square could be recorded at any of four intersections, but since our sightings were mostly obviously closer to one particular intersection, this random source of variation was rare. Also, this particular source of variation would tend to average out over many sightings.
We compared our home range sizes with those in the literature (Table 5). To our knowledge, these data are all that are available for the species in this study. Due to the large differences between the same species in the different studies and the use of various techniques to measure home range size, the data were compared using Spearman rank correlations (bootstrap estimation gave 95% confidence limits). The highest correlation (0.75; 95% confidence limits = 0.39–0.96) occurred between our data and data recorded by Marchant (1992) on the same site. Correlations with data from other authors were low (0.19–0.32), and their 95% confidence limits indicated they were not significantly different from zero (i.e., their 95% confidence limits spanned zero). Thus, there seemed to be no similarity between our ADCArea values and home range sizes from other sites (studies). However, the fact that home range sizes derived from two different studies (conducted approximately 30 years apart at the same site, and estimated using different methods [Marchant 1992]) were highly correlated suggests that differences from other studies could be the result of differences in habitats. It is essentially impossible to qualify the differences between the various habitats in the studies in Table 5. Microhabitats would differ for different species on the same site and could vary independently among sites; therefore, it is a complex issue. But there are several examples from both Australia and studies from other countries that suggest that habitat affects home range sizes. But the mechanisms would differ in each case, and in most cases are still to be elucidated (Ambrose and Davies 1989, Tidemann 1990, Chan and Augusteyn 2003, Stouffer 2007, Skorupski et al. 2018). Hence, the large variation in home range areas in the literature is perhaps not unexpected. Wide variations in home range sizes of species, both among and within studies, appear to be the rule, not the exception.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.
ACKNOWLEDGMENTS
We thank Anthony Overs for organizing the banding effort over the duration of the project.
DATA AVAILABILITY
All data are included in the manuscript.
LITERATURE CITED
Adams, E. S. 2001. Approaches to the study of territory size and shape. Annual Review Ecological Systems 32:277-303. https://doi.org/10.1146/annurev.ecolsys.32.081501.114034
Ambrose, S. J., and S. J. J. F. Davies. 1989. The social organisation of the White-browed Scrubwren Sericornis frontalis Gould (Acanthizidae) in arid, semi-arid and mesic environments of Western Australia. Emu 89:40-46. https://doi.org/10.1071/MU9890040
Anich, N. M., T. J. Benson, and J. C. Bednarz. 2009. Estimating territory and home-range sizes: Do singing locations alone provide an accurate estimate of space use? Auk 126:626-634. https://doi.org/10.1525/auk.2009.08219
Atua, F. A., and S. A. Manu. 2013. Territory size and habitat selection of Cinnamon-breasted Rock Bunting Emberiza tahapisi in Nigeria. Journal of African Ornithology 84:71-78. https://doi.org/10.2989/00306525.2013.777947
Austin, M. P. 1978. Vegetation. Pages 44-66 in R. H. Gunn, editor. Biophysical background studies, land use on the south coast of New South Wales. Volume 2. CSIRO, Melbourne, Australia.
Bell, H. L., and H. A. Ford. 1987. Fidelity to breeding site in four migratory species near Armidale, New South Wales. Corella 11:1-5.
Birkhead, T. 2008. The wisdom of birds. Bloomsbury, London, UK.
Bridges, L. 1994. Breeding biology of a migratory population of the Rufous Whistler Pachycephala rufiventris. Emu 94:106-115. https://doi.org/10.1071/MU9940106
Bureau of Meteorology. 2018. Southern Oscillation Index Archives—1876 to present. Australian Government.
Chan, K., and J. D. Augusteyn. 2003. Relationship between bird-unit size and territory quality in three species of fairy-wrens (Malurus spp) with overlapping territories. Ecological Research 18:73-80. https://doi.org/10.1046/j.1440-1703.2003.00534.x
Chaves, F. G., M. B. Vecchi, A. F. Kenup, and M. A. S. Alves. 2019. Territory size and population density of the Serra Antwren (Formicivora srrana littoralis) in the sandy coastal plains of the Atlantic Forest in southeastern Brazil. Annales Zoologici Fennici 56:51-64. https://doi.org/10.5735/086.056.0106
Clarke, M. F., C. Schipper, R. Boulton, and J. Ewen. 2003. The social organization and breeding behaviour of the Yellow-faced Honeyeater Lichenostomus chrosops – a migratory passerine from the Southern Hemisphere. Ibis 145:611-623. https://doi.org/10.1046/j.1474-919X.2003.00203.x
Colombelli-Négrel, D. 2016. Female Splendid and Variegated Fairy-wrens display different strategies during territory defence. Animal Behaviour 119:99-110. https://doi.org/10.1016/j.anbehav.2016.07.001
Debus, S. J. S. 2006. Breeding and population parameters of robins in a woodland remnant in northern New South Wales, Australia. Emu 106:147-156. https://doi.org/10.1071/MU04013
Duca, L., and M. A. Marini. 2014. Territorial system and adult dispersal in a cooperative-breeding tanager. Auk 131:32-40. https://doi.org/10.1642/AUK-13-005.1
Erickson, E. 1950. Inheritance of territory in Rufous Whistlers and notes on begging in courtship. West Australian Naturalist 2:145-150.
Fedy, B. C., and B. J. M. Stuchbury. 2005. Territory defence in tropical birds: Are females as aggressive as males? Behavioral Ecology and Sociobiology 58:414-422. https://doi.org/10.1007/s00265-005-0928-4
Green, D. J., and A. Cockburn. 1999. Life history and demography of an uncooperative Australian passerine, the Brown Thornbill. Australian Journal of Zoology 47:633-649. https://doi.org/10.1071/ZO99052
Green, D. J., and A. Cockburn. 2001. Post-fledging care, philopatry and recruitment in Brown Thornbills. Journal of Animal Ecology 70:505-514. https://doi.org/10.1046/j.1365-2656.2001.00503.x
Guppy, M., A. Overs, and S. Guppy. 2021. A detailed description of the breeding season of a community of birds on the south-east coast of Australia. Australian Zoologist 41:761-772. https://doi.org/10.7882/AZ.2021.014
Haché, S., M.-A. Villard, and E. M. Bayne. 2013. Experimental evidence for an ideal free distribution in a breeding population of a territorial songbird. Ecology 94:861-869. https://doi.org/10.1890/12-1025.1
Haila, Y., A. O. Nicholls, I. K. Hanski, and S. Raivio. 1996. Stochasticity in bird habitat selection: year-to-year changes in territory locations in a boreal forest bird assemblage. Oikos 76:536-552. https://doi.org/10.2307/3546347
Higgins, P. J., and J. M. Peter, editors. 2002. Handbook of Australian, New Zealand and Antarctic birds. Volume 6: pardalotes to shrike-thrushes. Oxford University Press, Melbourne, Australia.
Higgins, P. J., J. M. Peter, and W. K. Steele, editors. 2001. Handbook of Australian, New Zealand and Antarctic Birds. Volume 5: tyrant-flycatchers to chats. Oxford University Press, Melbourne, Australia.
Holmes, R. T. 2011. Avian population and community processes in forest ecosystems: long-term research in the Hubbard Brook Experimental Forest. Forest Ecology and Management 262:20-32. https://doi.org/10.1016/j.foreco.2010.06.021
Howard, H. E. 1920. Territory in bird life. Murray, London, UK.
Juárez, R., E. Chacón-Madrigal, and L. Sandoval. 2020. Urbanization has opposite effects on the territory size of two passerine birds. Avian Research 11:1-9. https://doi.org/10.1186/s40657-019-0187-0
Lack, D. 2015. The life of the robin. Pallas Athene, London, UK.
Langmore, N. E. 2000. Why female birds sing. Pages 317-327 in Y. Espmark, Y. T. Amundsen, and G. Rosenqvist, editors. Animal signals: signalling and signal design in animal communication. Tapir Academic Press.
Loyn, R. H. 1980. Bird populations in a mixed eucalypt forest used for production of wood in Gippsland, Victoria. Emu 80:145-156. https://doi.org/10.1071/MU9800145
Marchant, S. 1987. Territorialism and co-operative breeding of the Eastern Yellow Robin Eopsaltria australis. Corella 11:6-14.
Marchant, S. 1992. A bird observatory at Moruya, NSW 1975-84. Eurobodalla Natural History Society Occasional Publication No. 1.
Marchant, R., M. Guppy, and S. Guppy. 2021. The influence of the Southern Oscillation Index on the timing of breeding of a forest-bird community in south-eastern Australia. Wildlife Research 48:730-736. https://doi.org/10.1071/WR21004
Marchant, R., S. Guppy, and M. Guppy. 2016. The influence of ENSO and rainfall on the numbers of breeding pairs in a woodland bird community from south-eastern Australia. Emu 116:254-261. https://doi.org/10.1071/MU15087
Marshall, M. R., and R. J. Cooper. 2004. Territory size of a migratory songbird in response to caterpillar density and foliage structure. Ecology 85:432-445. https://doi.org/10.1890/02-0548
Mathias, L. B., and C. Duca. 2016. Territoriality of six Thanmohilidae species in a cloud forest in southeastern Brazil. Wilson Journal of Ornithology 128:752-759. https://doi.org/10.1676/15-184.1
Nakagawa, S., and H. Schielzeth. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution 4:133-142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Nicholls, N. 1991. The El Niño/Southern Oscillation and Australian vegetation. Vegetation 91:23-36. https://doi.org/10.1007/BF00036045
Noble, G. K. 1939. The role of dominance in the social life of birds. Auk 56:263-273. https://doi.org/10.2307/4079047
Noske, R. A. 1991. A demographic comparison of cooperatively breeding and non-cooperative treecreepers (Climacteridae). Emu 91:73-86. https://doi.org/10.1071/MU9910073
Odum, K. J., E. M. Rose, M. T. Hallworth, O. A. Díaz-Marrero, and K. E. Omland. 2019. Females and males maintain similar-sized, stable territories between breeding and nonbreeding seasons in a Tropical Oriole (Icterus ictertus). Wilson Journal of Ornithology 131:524-533. https://doi.org/10.1676/18-135
Ottaviani, D., S. C. Cairns, M. Oliverio, and L. Boitani. 2006. Body mass as a predictive variable of home-range size among Italian mammals and birds. Journal of Zoology 269:317-330. https://doi.org/10.1111/j.1469-7998.2006.00060.x
Schieck, J. O., and S. J. Hannon. 1993. Clutch predation, cover, and the overdispersion of nests of the Willow Ptarmigan. Ecology 74:743-750. https://doi.org/10.2307/1940802
Seastedt, T. R., and S. F. MacLean. 1979. Territory size and composition in relation to resource abundance in Lapland Longspurs breeding in arctic Alaska. Auk 96:131-142.
Skorupski, J., L. Jankowiak, L. B. Kiriaka, T. Rek, and D. Wysocki. 2018. Beech forest structure and territory size of four songbird species in Puszcza Bukowa, NW Poland: implications for bird-friendly silviculture practices in a temperate forest. Ethology Ecology & Evolution 30:128-140. https://doi.org/10.1080/03949370.2017.1329232
Stouffer, P. C. 2007. Density, territory size, and long-term spatial dynamics of a guild of terrestrial insectivorous birds near Manaus, Brazil. Auk 124:291-306. https://doi.org/10.1093/auk/124.1.291
Tidemann, S. C. 1990. Factors affecting territory establishment, size and use by three co-existing species of fairy-wrens (Malurus). Emu 90:7-14. https://doi.org/10.1071/MU9900007
Van Dongen, W. F. D., and L. L. Yocom. 2005. Breeding biology of migratory Australian passerine, the Golden Whistler (Pachycephala pectoralis). Australian Journal of Zoology 53:213-220. https://doi.org/10.1071/ZO04081
Vargas, L. E., N. V. Sánchez, and G. Avalos. 2011. Forest structure and territory size relationship in the neotropical understorey insectivore Henicorhina leucosticte. Journal of Tropical Ecology 27:65-72. https://doi.org/10.1017/S026646741000060X
Yoon, J. 2014. Predicting territory density of Dusky Orange-crowned Warblers Oreothlypis celata sordida breeding on Santa Catalina Island, California. Bird Study 61:474-483. https://doi.org/10.1080/00063657.2014.950551
Zanette, L. 2000. Fragment size and the demography of an area-sensitive songbird. Journal of Animal Ecology 69:458-470. https://doi.org/10.1046/j.1365-2656.2000.00408.x
Fig. 1
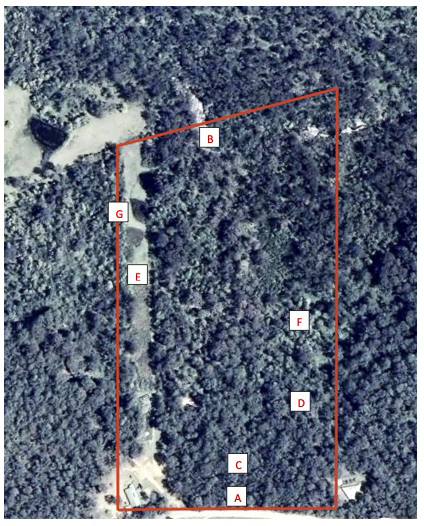
Fig. 1. Aerial view of the study site. North is to the right, the site is outlined in red, and the dwelling of authors MG and SG is at the bottom left-hand corner. The boxed letters are referred to in the Methods.

Fig. 2
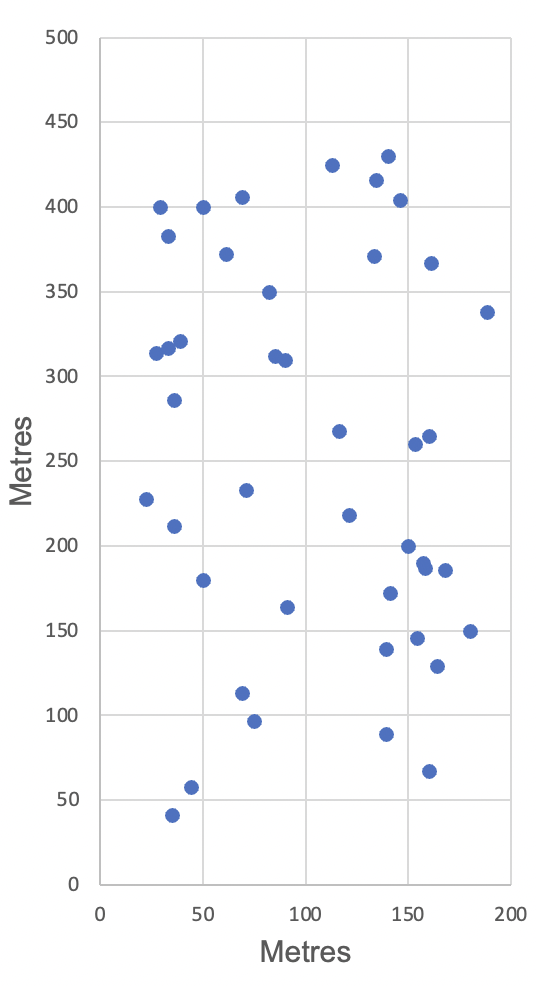
Fig. 2. Centroids of all calculated male home ranges during the 2010–2011 season. The data represent three Eastern Yellow Robins, 10 Brown Thornbills, one Variegated Fairy-wren, one Eastern Spinebill, one Golden Whistler, three Rufous Whistlers, one Lewin’s Honeyeater, three White-browed Scrubwrens, 10 Superb Fairy-wrens, and 10 Yellow-faced Honeyeaters. Not all home ranges are represented because there were not enough sightings for all individuals. Any home range with a centroid less than 20 m from a boundary was not included in the analysis. The orientation of the site is the same as in Fig. 1.

Fig. 3
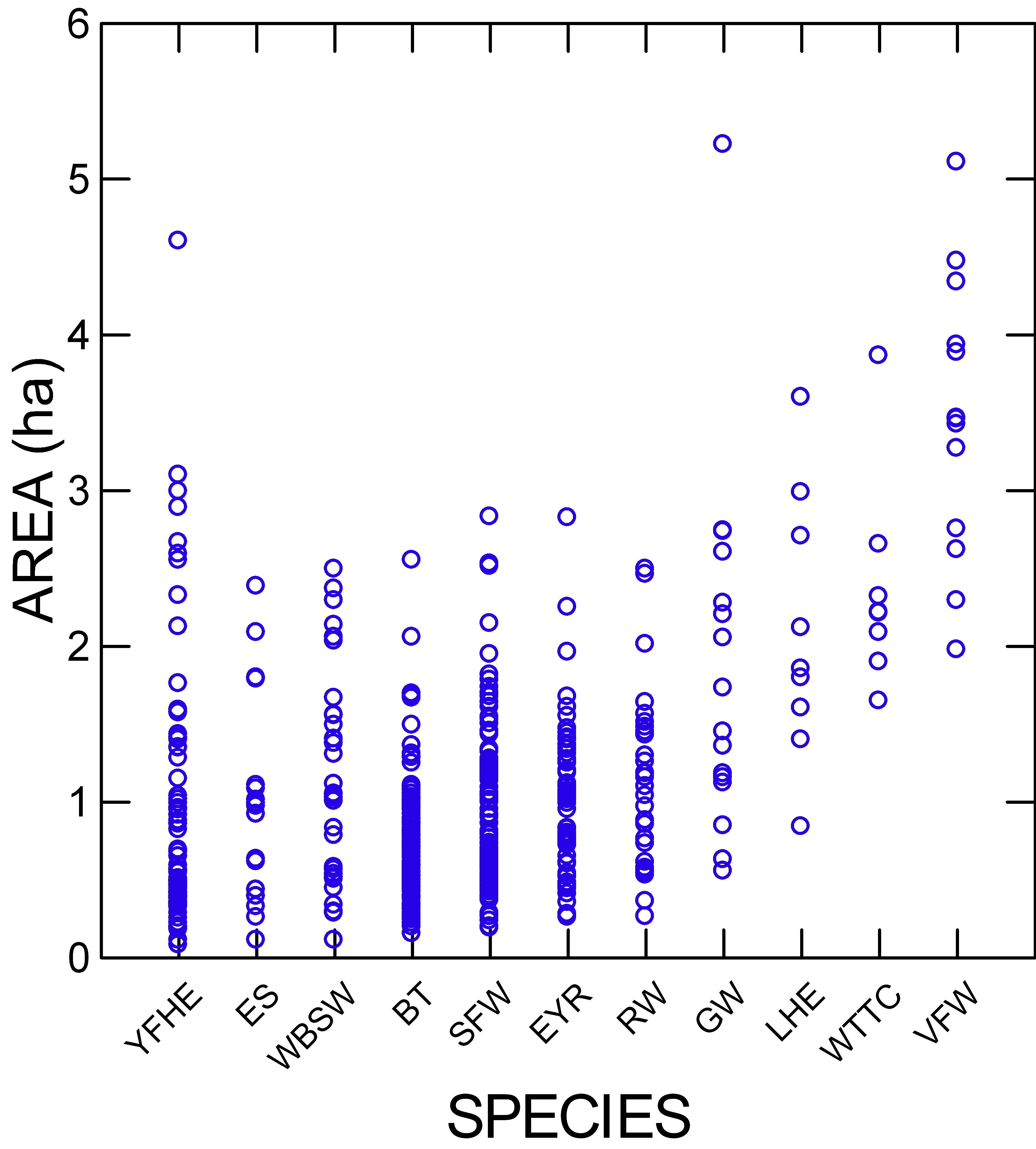
Fig. 3. Range of home range (ADCArea) sizes for each species. (YFHE = Yellow-faced Honeyeater; ES = Eastern Spinebill; WBSW = White-browed Scrubwren; BT = Brown Thornbill; SFW = Superb Fairy-wren; EYR = Eastern Yellow Robin; RW = Rufous Whistler; GW = Golden Whistler; LHE = Lewin’s Honeyeater; WTTC = White-throated Treecreeper; VFW = Variegated Fairy-wren).

Fig. 4
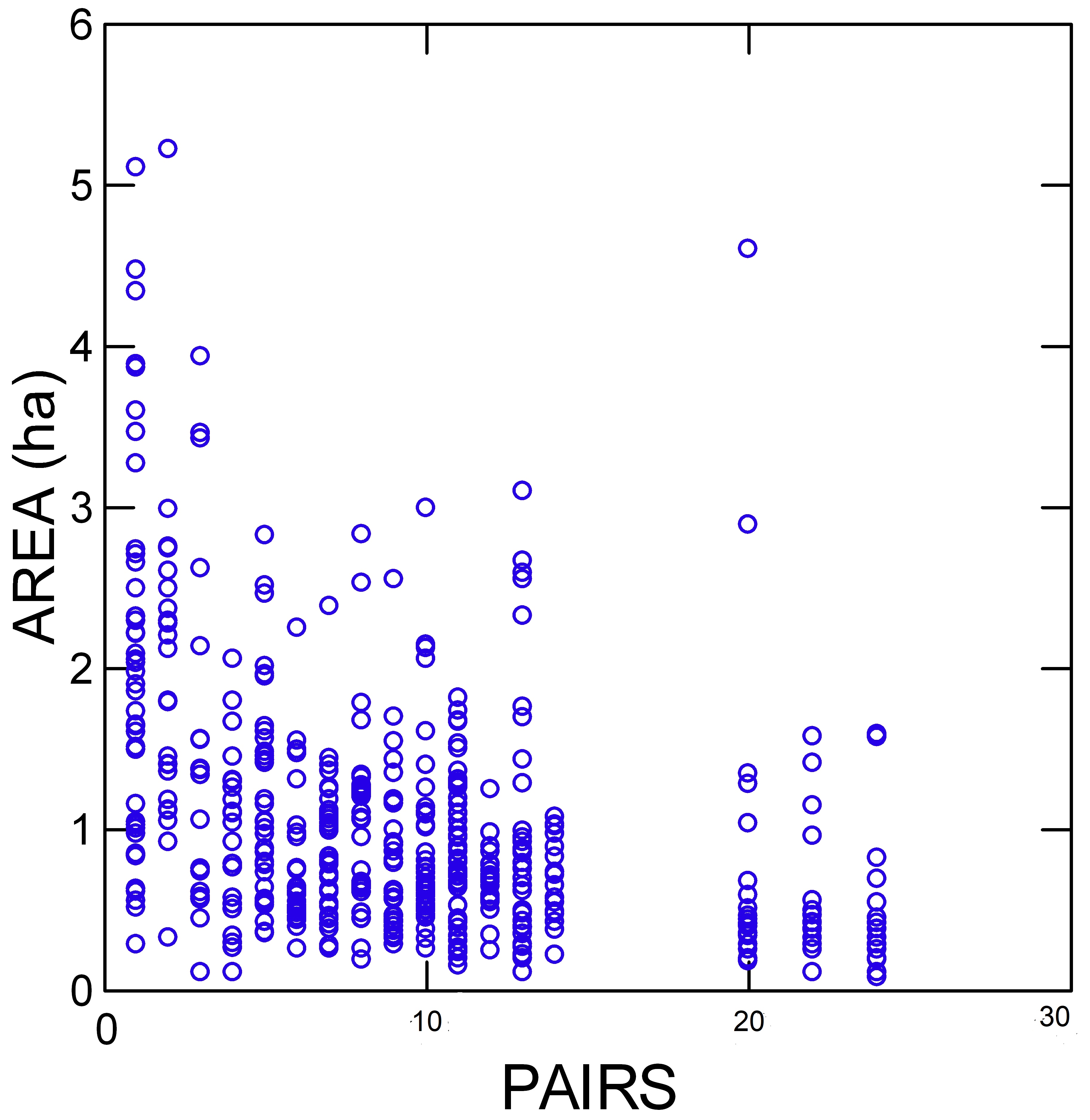
Fig. 4. Home range sizes versus number of pairs. Each point represents one individual of any of the 11 species, for a single breeding season. For example, the point at X = 10, Y = 3 represents an individual of one of the 11 species that had a home range area of 3 ha in a season in which there were 10 pairs of that species.

Fig. 5
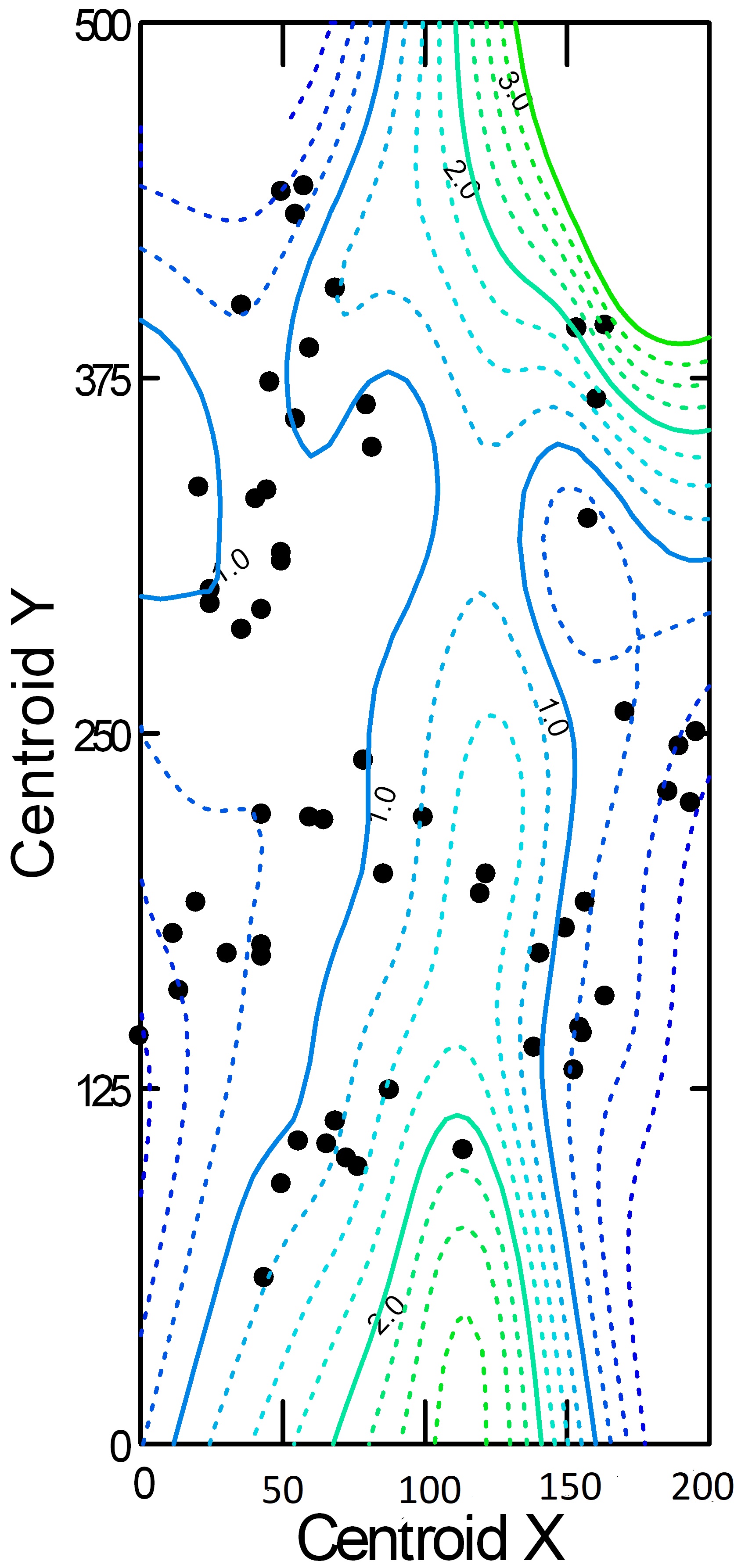
Fig. 5. Contour map for the male Superb Fairy-wren showing the territory centroids (black filled circles) over all years. As in Fig. 2, not all individuals breeding on the site are represented. The numbers on the contours represent ADCAreas (ha), and the colors of the contours grade from blue through light green to dark green as ADCAreas increase. The area of commonality mentioned in the text is centred on X = 150, Y = 375.

Table 1
Table 1. Study species, the total number of individuals included in the study (N), the mean number of sightings per individual per season (MS), and the total number of sightings for that species over the study (TS). A range of weights (g) is given after each species name.
| Species | N† Male Female |
MS (SD) Male Female |
TS† |
| White-throated Treecreeper (Cormobates leucophaeus) (16–24) |
4 4 |
20.0 (3.4) 20.0 (2.2) |
80 80 |
| Superb Fairy-wren (Malurus cyaneus) (9–15) |
64 36 |
12.0 (5.3) 12.4 (5.7) |
768 446 |
| Variegated Fairy-wren (Malurus lamberti) (6–11) |
9 5 |
14.0 (4.5) 15.0 (7.2) |
126 75 |
| White-browed Scrubwren (Sericornis frontalis) (10–19) |
18 12 |
12.0 (5.4) 6.9 (1.8) |
216 83 |
| Brown Thornbill (Acanthiza pusilla) (5–8) |
69 43 |
14.0 (6.0) 12.0 (5.0) |
966 516 |
| Eastern Spinebill (Acanthorhynchus tenuirostris) (8–16) |
8 10 |
6.8 (3.2) 8.1 (4.6) |
54 81 |
| Lewin’s Honeyeater (Meliphaga lewinii) (27–49) |
5 4 |
8.0 (2.3) 10.0 (2.4) |
40 40 |
| Yellow-faced Honeyeater (Lichenostomus chrysops) (15–20) |
49 44 |
7.0 (3.0) 5.9 (2.0) |
343 260 |
| Eastern Yellow Robin (Eopsaltria australis) (15–27) |
36 27 |
17.0 (10.7) 12.4 (4.5) |
612 335 |
| Golden Whistler (Pachycephala pectoralis) (25–35) |
8 8 |
11.0 (3.6) 12.1 (3.5) |
88 97 |
| Rufous Whistler (Pachycephala rufiventris) (18–32) |
16 13 |
9.0 (3.4) 6.4 (2.9) |
144 83 |
| †This can include multiple entries for one individual if it was on the site for more than one season. The total number of sightings was calculated by multiplying N by MS. | |||
Table 2
Table 2. Home range sizes measured as average distance of sightings from the centroid (ADC) for males and females.
| Species | Average male ADC† (m) | Range of male ADC | Average female ADC† (m) | Range of female ADC | Average area for males (ha)‡ |
| White-throated Treecreeper | 81.8 | 77.7–84.0 | 90.3 | 72.4–110.9 | 2.10 |
| Superb Fairy-wren | 54.2 | 24.5–94.9 | 49.8 | 25.0–89.7 | 0.92 |
| Variegated Fairy-wren | 105.3 | 79.3–127.5 | 96.9 | 79.3–105.0 | 3.46 |
| White-browed Scrubwren | 62.3 | 32.7–86.8 | 50.8 | 18.8–89.1 | 1.20 |
| Brown Thornbill | 49.1 | 25.0–90.1 | 45.1 | 22.0–68.9 | 0.76 |
| Eastern Spinebill | 46.3 | 28.6–59.3 | 54.5 | 18.8–87.1 | 0.67 |
| Lewin’s Honeyeater | 80.1 | 51.7–107.0 | 80.3 | 71.4–97.5 | 2.00 |
| Yellow-faced Honeyeater | 45.0 | 24.5–95.9 | 47.8 | 16.0–121.0 | 0.64 |
| Eastern Yellow Robin | 55.8 | 28.6–73.0 | 52.6 | 29.7–94.8 | 0.98 |
| Golden Whistler | 68.5 | 42.0–93.4 | 79.5 | 51.9–128.9 | 1.50 |
| Rufous Whistler | 60.1 | 28.9–89.1 | 56.0 | 33.9–88.5 | 1.10 |
| †The average of all the ADCs calculated separately for each individual of each species. The data can include multiple entries for one individual if it was on the site for more than one season. ‡Area calculated using male ADC values as the radius of a circle. Areas have been converted to hectares for comparison with values in the literature. | |||||
Table 3
Table 3. Linear mixed models for log10 ADCArea. Species and sex were fixed factors; year, pairs, and average Southern Oscillation Index for April to July (AJSOI) were fixed covariates. Year ID was included as a random factor. Number of observations = 490; DF = 470–472. R2 is the variance explained by the fixed factors.
| Model | Estimated effect | AIC corrected† | Δ AIC | t | P | R2 |
| 1: species | 142.4 | 0.8 | 4.24 | < 0.001 | 0.26 | |
| 2: species | 144.4 | 2.8 | 4.25 | < 0.001 | 0.27 | |
| + sex | -0.046 | -1.89 | 0.060 | |||
| 3: species | 141.6 | 0 | 2.63 | < 0.001 | 0.29 | |
| + sex | -0.046 | -1.90 | 0.060 | |||
| + pairs | -0.015 | -3.60 | 0.003 | |||
| 4: species | 144.1 | 2.5 | 2.67 | < 0.001 | 0.30 | |
| +sex | -0.047 | -1.92 | 0.060 | |||
| +year | -0.018 | -2.73 | 0.006 | |||
| + pairs | -0.015 | -3.40 | <0.001 | |||
| 5: species | 154.3 | 12.7 | 2.66 | < 0.001 | 0.30 | |
| + sex | -0.047 | -1.92 | 0.060 | |||
| + year | -0.018 | -2.68 | 0.008 | |||
| + pairs | -0.015 | -3.41 | 0.0007 | |||
| + AJSOI | 0.001 | 0.52 | 0.604 | |||
| †AIC corrected = corrected Akaike information criterion. | ||||||
Table 4
Table 4. Raw ADC (m) values and centroid co-ordinates for individual males of some species. Each row is a different season for each species. (a) and (b) represent two different males, each of which had a different female partner. The co-ordinates of the territory centroids for each season were used to calculate a master centroid of the territory centroids and the average distance of all territory centroids from the master centroid.
| Species | ADC† | Centroid X | Centroid Y | Average distance of territory centroids from the master centroid (m) |
| White-throated Treecreeper (a) | 82 | 108 | 87 | 15.3 |
| 83 | 134 | 103 | ||
| White-throated Treecreeper (b) | 78 | 143 | 102 | 17.4 |
| 84 | 111 | 113 | ||
| Superb Fairy-wren | 45 | 50 | 310 | 18.9 |
| 54 | 41 | 332 | ||
| 76 | 36 | 286 | ||
| Brown Thornbill | 39 | 154 | 465 | 12.9 |
| 59 | 150 | 438 | ||
| 90 | 157 | 436 | ||
| Yellow-faced Honeyeater | 52 | 39 | 321 | 12.0 |
| 33 | 14 | 321 | ||
| 42 | 20 | 310 | ||
| 67 | 25 | 300 | ||
| †ADC = average distance of all sightings from the centroid. | ||||
Table 5
Table 5. Home range areas from the literature.
| Species | Area (ha)† | Reference | Sample size |
| White-throated Treecreeper | 1.4 | Loyn 1980‡ | One breeding season |
| 2.1 | This study | ||
| 3.9–5.8 | Noske 1991 | n = 14 | |
| 6.0 | Marchant 1992§ | Estimates based on 10 years of observation | |
| Superb Fairy-wren | 0.50–0.75 | Marchant 1992 | |
| 0.92 | This study | ||
| 1.5 | Loyn 1980 | ||
| 1.3–2.3 | Tidemann 1990 | n = 6–12 over four seasons | |
| 3.5–16.0 | Chan and Augusteyn 2003 | n = 9 | |
| Variegated Fairy-wren | 3.5 | This study | |
| 3.8 | Tidemann 1990 | ||
| 3.5–10.0 | Chan and Augusteyn 2003 | ||
| 4.0 | Marchant 1992 | ||
| White-browed Scrubwren | 0.7 | Loyn 1980 | |
| 1.2 | This study | ||
| 1.3–2.5 | Marchant 1992 | ||
| 1.4–2.6 | Ambrose and Davies 1989 | n = 10 | |
| Brown Thornbill | 0.4–3.1 | Green and Cockburn 1999 | n = 136 over three seasons |
| 0.4 | Loyn 1980 | ||
| 0.5–0.8 | Marchant 1992 | ||
| 0.8 | This study | ||
| 2.0 | Green and Cockburn 2001 | n = 30 pairs each season for four seasons | |
| Eastern Spinebill | 0.7 | This study | |
| 2.1 | Loyn 1980 | ||
| 2.0–3.0 | Marchant 1992 | ||
| Lewin’s Honeyeater | 2.0 | This study | |
| 4.1 | Loyn 1980 | ||
| Yellow-faced Honeyeater | 0.2 | Clarke et al. 2003 | n = 11 |
| 0.6 | This study | ||
| 1.0 | Marchant 1992 | ||
| 1.2 | Loyn 1980 | ||
| Eastern Yellow Robin | 1.0 | This study | |
| 1.1 | Loyn 1980 | ||
| 0.8–2.0 | Marchant 1992 | ||
| 2.5–5.5 | Zanette 2000 | n = 39–72 over three seasons | |
| 5.0–6.0 | Debus 2006 | n = 8–11 over three seasons | |
| Golden Whistler | 0.8 | Loyn 1980 | |
| 1.5 | This study | ||
| 2.5 | Van Dongen and Yocom 2005 | n = 71 | |
| 4.5–5.0 | Marchant 1992 | ||
| Rufous Whistler | 1.1 | This study | |
| 1.0–3.0 | Bell and Ford 1987 | n = 3 over two seasons | |
| 1.2–4.2 | Bridges 1994 | n = 96 over four seasons | |
| 3.0 | Loyn 1980 | ||
| 3.0 | Marchant 1992 | ||
| †Areas were determined mostly (but not always) during the breeding season, using a variety of methods; i.e., densities of unbanded birds, positions of singing birds, and positions of unbanded and color-banded birds. ‡All areas from this author were calculated using species densities. §These data are from a study done on the same site between 1975 and 1984. | |||








