The following is the established format for referencing this article:
Bakner, D. L., K. E. Miranda, and K. M. Ringelman. 2022. Louisiana Black-bellied Whistling-Duck clutch characteristics in the presence of conspecific and interspecific brood parasitism. Journal of Field Ornithology 93(4):8.ABSTRACT
Black-bellied Whistling-Ducks (Dendrocygna autumnalis; Whistling-Duck) are undergoing a rapid range expansion northward and now breed throughout the Southeastern United States. As a facultative cavity-nesting species, they have the potential to compete with Wood Ducks (Aix sponsa) and Hooded Mergansers (Lophodytes cucullatus) for nest sites. Little is known about Whistling-Duck breeding biology, and estimates of clutch characteristics and rates of conspecific and interspecific brood parasitism (CBP and IBP, respectively) are lacking. We monitored Whistling-Duck nests in Louisiana to describe nesting chronology, clutch size of parasitized and unparasitized (normal) nests, and hatchability (i.e., the portion of eggs that hatched) for clutches of different sizes and types. We monitored a total of 558 nest boxes between 2020–2021 and determined the presence of brood parasitism for 231 Whistling-Duck nests. CBP was detected in 73 (31.6%) nests, and IBP was observed in 51 (22.1%) nests parasitized by Wood Ducks, two (0.9%) nests parasitized by Hooded Mergansers, and one nest contained eggs from all three species. Normal clutches were smaller (15.4 ± 4.4 eggs) than CBP clutches (26.1 ± 8.8 eggs) and mixed clutches (22.2 ± 5.3 eggs; clutches containing Wood Duck or Hooded Merganser eggs; all pairwise P < 0.0001). However, within clutch repeatability, estimates for egg morphology data (i.e., length, width, and mass) were low (< 0.40) for normal clutches, suggesting CBP went undetected. Of 180 fated nests used to determine hatchability, 66 (36.7%) were successful, 49 (27.2%) were abandoned, 64 (35.6%) were depredated, and one (0.6%) was nonviable. Considering successful nests, hatchability was high for all clutch size bins ranging from 67.4% (41–45 eggs) to 81.6% (11–15 eggs). Our study is the first to document Whistling-Ducks successfully hatching mixed-species broods, and such high productivity could be contributing to Whistling-Duck range expansion.RESUMEN
INTRODUCTION
The Black-bellied Whistling-Duck (Dendrocygna autumnalis; Whistling-Duck) is primarily a neotropical waterfowl species historically distributed from northern South America to Southern Texas (James and Thompson 2020). However, their range began expanding northward in the middle part of the 20th century, with individuals commonly sighted in Arizona, Louisiana, and Florida by the 1960s (Baldassarre 2014). By the early 2000s, breeding populations had also been established in Arkansas, Oklahoma, Tennessee, and South Carolina (Bergstrom 1999, Baldassarre 2014, Cohen et al. 2019, Croft et al. 2020), and since then, Whistling-Ducks have been documented breeding as far north as Wisconsin and Nebraska, and pioneering individuals observed as far north as central Quebec (https://ebird.org/home).
Whistling-Ducks are generalists and can flourish in a wide variety of habitats including wetlands, agricultural fields, stockyards, and urban areas (Bourne 1981, Matta et al. 2014, Cohen et al. 2019), which has undoubtedly contributed to their northward range expansion. Similarly, Whistling-Ducks will use a variety of nesting strategies and can nest in natural cavities, artificial nest structures (nest boxes), or on the ground (Bolen et al. 1964, McCamant and Bolen 1979, Markum and Baldassarre 1989, Edmonds and Stolley 2008). Thus, as facultative cavity-nesters, Whistling-Ducks have the potential to interact and compete for nest sites with other cavity nesting species, such as the Wood Duck (Aix sponsa). Croft et al. (2020) identified substantial niche overlap in both nest box preferences and timing of nesting in South Carolina, where Wood Ducks nested January to July and Whistling-Ducks nested May to September. Given the temporal overlap in nest box use (Croft et al. 2020) and that both species are conspecific brood parasites (McCamant and Bolen 1979, Semel et al. 1988, James 2000), there is potential for interspecific brood parasitism (IBP) to occur. A single instance of Whistling-Ducks parasitizing Wood Duck nests has been documented in the literature based on two mixed clutches that were incubated by Wood Ducks (Bolen and Cain 1968); there are no publications that document Wood Ducks laying parasitic eggs in Whistling-Duck nests. Additionally, IBP between Whistling-Ducks and Hooded Mergansers (Lophodytes cucullatus) is possible as the latter can initiate nests as late as April (Baldassarre 2014).
Whereas the frequency and fitness implications of IBP between Whistling-Ducks and other species are unknown, conspecific brood parasitism (CBP) has been described through nest box studies (Delnicki 1973, McCamant and Bolen 1979). In Whistling-Ducks, the prevalence of CBP varies by study, because eggs have not been genetically vetted, and researchers have selected different clutch size thresholds to demarcate eggs laid by a single female (Delnicki 1973, James 2000). However, parasitism generally results in larger clutch sizes, which increases the odds of nest abandonment and decreases the portion of eggs that hatch (hatchability; Delnicki 1973, McCamant and Bolen 1979). For example, McCamant and Bolen (1979) observed CBP in 70% of Whistling-Duck nests and found the hatchability of 21,982 eggs was only 20% because of high rates of nest abandonment.
The paucity of research on Whistling-Duck breeding ecology is noteworthy, given their rapid expansion and potential competitive and parasitic interactions with Wood Ducks. Insufficient information exists regarding Whistling-Duck ecology to predict how their range expansion and parasitic behavior may affect native waterfowl species and thereby prompt management actions. Here, our objectives were to monitor Whistling-Duck nests to (1) describe nesting chronology as it overlaps with sympatric nesting duck species, (2) estimate rates of CBP and IBP, (3) estimate clutch size for nests with CBP and IBP, and (4) estimate hatchability for clutches of different sizes and types (i.e., parasitized and non-parasitized clutches).
METHODS
Study sites
We monitored nest boxes erected by the Louisiana Department of Wildlife and Fisheries (LDWF) for Wood Duck use. Our study areas were Sherburne Wildlife Management Area (Sherburne), Thistlethwaite Wildlife Management Area (Thistlethwaite), Indian Creek Reservoir, Lake Rodemacher, and Oden Lake (Fig. 1). Sherburne is in Iberville, Pointe Coupee, and St. Martin Parishes, 17,652 ha in size, and owned by LDWF (4775 ha), U.S. Fish and Wildlife Service (6159 ha), and U.S. Army Corps of Engineers (6725 ha). Adjacent to the Atchafalaya River, Sherburne is primarily a bottomland hardwood forest with several backswamps and bayous. Two eastern portions of Sherburne known as “North Farm” and “South Farm” are managed as moist soil impoundments for waterfowl, shorebirds, and wading birds. Thistlethwaite is a 4492 ha privately owned, bottomland hardwood forest within St. Landry parish that is managed by LDWF. As part of the Thistlethwaite study area, we oversaw nest boxes at the St. Landry Parish Solid Waste Disposal District (disposal district). Located ~1.5 km northeast of Thistlethwaite, the disposal district constructed a pond and installed nest boxes for Wood Ducks as part of a mitigation project. Indian Creek is a 1052 ha reservoir located in Rapides Parish. Alexander State Forest Wildlife Management Area surrounds the reservoir. Patches of loblolly (Pinus taeda) and longleaf pine (Pinus palustris) stands can be found scattered throughout hardwood stands that line creek drainages. Oden Lake (Rapides Parish) is privately owned and located ~6.5 km northeast of Indian Creek, partially surrounded by residential housing. As part of the Oden Lake study site, we monitored nest boxes located in a cypress swamp directly north of the lake and west of highway 165. Lake Rodemacher is 1189 ha in size and located in Rapides Parish and ~3 km west of Boyce, Louisiana. The Brame Energy Center is located on the northwest side of the lake and uses its water as a cooling resource when generating power. Nest boxes were located over open water at sites accessed by boats and off the side of levees in areas that could be navigated by all-terrain vehicles and on foot.
During 2020, we monitored 285 nest boxes, consisting of 236 duplexes (two boxes mounted on either side of a pole) and 49 single units. To increase sample size in 2021, we converted 10 single units to duplexes at both North and South Farm and started overseeing 10 duplexes at Lake Rodemacher. Considering these additions, we increased our nest box sample size to 325, consisting of 296 that were arranged in duplexes and 29 single units. All nest boxes had conical sheet metal predator guards.
Nest monitoring and clutch descriptions
We visited nest boxes at approximately seven-day intervals in 2020 and 2021. We determined nest initiation dates by back calculating to the day when the first egg was laid, assuming a laying rate of one egg per day with no partial clutch losses (Delnicki 1973, Emery et al. 2005). During weekly visits, we determined whether nests were active or terminated. We considered nests to be active if new eggs were added since the prior visit, egg incubation progressed (Weller 1956), or by observing a bird incubating the clutch. We classified terminated nests as being abandoned, depredated, or successful. We considered a nest abandoned if we observed egg laying or incubation discontinue without sign of clutch loss between two consecutive visits. Otherwise, we classified nests as depredated when eggs went missing or were destroyed and egg laying or incubation ceased. We considered a nest successful if it survived to hatch ≥ 1 egg; we counted unhatched eggs and counted egg membranes to determine the number of eggs that hatched.We assigned all eggs from each nest a numeric ID written with a permanent marker as they appeared in the nest (Semel et al. 1988). We determined the species of each egg and measured the length, width, and mass. We measured egg length and width to the nearest 0.1 mm using a dial caliper, and mass to the nearest 0.1 g using a digital pocket scale. Because of logistical restraints, we were unable to collect egg morphology data at Sherburne and the disposal district. There is no published literature to aid in distinguishing Whistling-Duck from Wood Duck eggs; therefore, we developed our own protocols to differentiate between the two species using egg candling techniques (Weller 1956). When viewed through a candling device, Wood Duck eggshells were transparent, whereas Whistling-Duck eggs showed a distinct blotchy pattern (Fig. 2). The blotchy pattern persisted throughout the incubation period, but was more difficult to view during later stages; however, we began monitoring most nests during laying or early incubation facilitating species identification. We used egg morphology and color to distinguish Hooded Merganser eggs (they are very round and white) from Wood Ducks and Whistling-Ducks (Mallory and Weatherhead 1990, Baldassarre 2014).
We categorized clutches based on the presence or absence of CBP and IBP. Whistling-Duck nests with CBP present (CBP clutches) received > 1 Whistling-Duck egg per day during the laying stage (MacWhirter 1989) and/or additional eggs following day four of incubation, as Whistling-Ducks begin incubation ~3 days prior to laying the last egg (Delnicki 1973). Whistling-Duck nests with IBP via Wood Ducks or Hooded Mergansers were termed “mixed clutches.” We found CBP was also present in some mixed clutches; we assigned these as mixed clutches. Nests not assigned to the CBP or mixed clutch types were termed “normal clutches.” We compared clutch sizes for different clutch types using t-tests and all measures reported are means ± standard deviation unless otherwise specified.
Detecting CBP
Because of the potential for CBP to go undetected in clutches designated “normal” based on the previous criteria, we evaluated egg morphology in an effort to verify that normal clutches were produced by a single female. We conducted a one-way analysis of variance (ANOVA) for each egg measurement to compare within- and between-clutch variability for normal and CBP clutches. We performed ANOVA tests in R version 4.1.3 using the aov() function (R Core Team 2021). We expected between-clutch variability to be greater than within-clutch variability because individual ducks lay eggs that are consistent in shape and size (Pöysä et al. 2009, Eadie et al. 2010, Lemons et al. 2011). To verify that greater variability between clutches was because of individuals laying morphologically consistent eggs, we estimated repeatability (Lessells and Boag 1987). We considered egg measurements to be characteristic of individual female Whistling-Ducks if repeatability estimates were moderate (0.4–0.7; Harper 1994), while estimates < 0.4 suggest CBP was present. When analyzing egg measurements, we excluded clutches containing less than three eggs, following James (2000) who used egg morphology to quantify CBP in Whistling-Duck nests.
Hatchability
We calculated hatchability for different clutch sizes and types by taking the total number of eggs laid in a given nest-species cohort and dividing by the total number that hatched (Semel et al. 1988). We considered incubated and non-incubated clutches when estimating hatchability. To achieve appropriate sample sizes for hatchability estimates, we binned clutches in five-egg increments (e.g., the first bin contained clutches ranging from one to five eggs, the second ranged from six to 10 eggs; McCamant and Bolen 1979). Because hatchability can vary seasonally with increasing temperatures (Hepp et al. 2006, DuRant et al. 2010), we produced monthly estimates of hatchability for different clutch types by grouping nests based on the month they were initiated.
RESULTS
We monitored nest boxes from 1 February until 28 July in 2020 and 2021. Whistling-Ducks began initiating nests in early spring (21 March and 11 April in 2020 and 2021) and continued through the end of July, when financial limitations forced us to cease monitoring boxes. Across both years, we observed new nests initiated over a span of 117.5 ± 14.8 days. There was no strong peak in nest initiation date; however, most nest initiation occurred during June (Fig. 3; Table 1). We monitored a total of 261 Whistling-Duck nests (126 and 135 nests in 2020 and 2021, respectively) that contained 4569 eggs. Of those nests, we determined the clutch type for 231 (88.5%). We observed 105 (45.5%) normal clutches, 73 (31.6%) CBP clutches, 51 (22.1%) mixed clutches parasitized by Wood Ducks, and two (0.9%) by Hooded Mergansers. Additionally, we observed CBP in 27 clutches assigned to the mixed clutch category. Considering these nests, we observed CBP in 99 (42.9%) clutches.
We measured 1858 (41.4%) Whistling-Duck eggs from 97 individual nests. Before analyzing egg measurements, we excluded 496 eggs from 23 mixed clutches and 13 eggs from nine normal clutches containing < 3 eggs; therefore, we considered 1349 eggs from 65 nests. CBP was present in 44 (67.7%) nests containing 1108 (82.1%) eggs and was absent in 21 (32.3%) nests containing 241 (17.9%) eggs. For all eggs considered, the mean length was 52.2 ± 2.0 mm, width was 38.6 ± 1.2 mm, and mass was 43.0 ± 3.6 g. Our ANOVA results showed more variation in egg morphology between clutches compared to within (all pairwise P < 0.0001; Table 2). Repeatability estimates were lower for nests with CBP and higher for normal clutches. However, repeatability never exceeded 0.40, which suggests that Whistling-Duck nests do not contain eggs consistent in size and shape; therefore, CBP was likely present in some clutches classified as normal.
A total of 126 nests were incubated, and we determined the clutch type for 105; these were used to describe clutch sizes throughout the nesting season (Table 1). The average clutch size of all incubated nests was 21.4 ± 8.2 and decreased throughout the nesting season (Fig. 4). Normal clutches averaged 15.4 ± 4.4 eggs (n = 38 nests). When compared to the mean clutch size of all incubated nests, normal clutches were significantly smaller (t = -5.561, df = 120.470, P < 0.0001). CBP clutches averaged 26.1 ± 8.8 eggs (n = 44 nests), which was significantly larger than normal clutches (t = -7.116, df = 65.347, P < 0.0001). Mixed clutches averaged 22.2 ± 5.3 eggs (n = 23 nests), which is also significantly larger than normal clutches (t = -5.162, df = 40.152, P < 0.0001).
We used 180 nests with determined fates to estimate hatchability. For the nests with determined fates, 66 (36.7%) were successful, 49 (27.2%) were abandoned, 64 (35.6%) were depredated, and one (0.6%) was nonviable. Hatchability averaged 29.8% across both years and was higher in 2020 (37.4%) compared to 2021 (22.2%). Hatchability was greatest (45.5%) for clutch sizes ranging from 31–35 and least (0.0%) for clutches ranging from 1–5 and 6–10 (Table 3). We found no clear relationship between clutch size and hatchability. Considering only successful nests, hatchability was greatest (81.6%) for clutch sizes ranging from 11–15 and least (24.3%) for clutches ranging from 51–55. Successful clutches smaller than the mean size of all incubated nests (x̅ = 21.4 ± 8.2, n = 105) had higher hatchability than those exceeding the mean. Of the nests with determined fates, we assigned a clutch type to 164, which we used to produce monthly hatchability estimates for each clutch type (Table 4). The overall hatchability of CBP clutches was 34.1%, which exceeded estimates for normal (26.7%) and mixed clutches (25.5%). We did not derive monthly estimates for clutches initiated in July, as they were projected to hatch following the conclusion of our field season. Hatchability was highest in April for normal (33.8%) and CBP clutches (43.2%), and June for mixed clutches (59.9%; Table 4).
A total of 1038 eggs successfully hatched; 973 (93.7%) were Whistling-Ducks, 63 (6.1%) were Wood Ducks, and two (0.2%) were Hooded Mergansers. Normal clutches hatched 135 (13.0%) Whistling-Ducks, CBP clutches hatched 588 (56.6%), and mixed clutches hatched 190 (18.3%). The remaining 125 (12.0%) eggs hatched from either a normal or CBP clutch (we were unable to determine the clutch type). We observed 15 successful mixed clutches, with each containing an average of 3.9 ± 2.9 Wood Duck ducklings and 11.9 ± 7.3 Whistling-Duck ducklings. Normal clutches hatched an average of 12.2 ± 5.5 ducklings (n = 11 nests) and CBP clutches averaged 16.3 ± 7.0 ducklings per nest (n = 36 nests).
DISCUSSION
This is the largest evaluation of Whistling-Duck nesting ecology to date, and the first to document Whistling-Ducks incubating and successfully hatching mixed clutches of Wood Ducks and Hooded Mergansers. Whistling-Ducks initiated nests earlier in Louisiana when compared to other breeding populations in the Southeastern U.S. (Bolen 1967, Croft et al. 2020), and sympatric nesting with Wood Ducks occurred for greater than three months, with IBP found in ~20% of nests. The majority of hatched eggs were Whistling-Duck, with < 7% being a Wood Duck or Hooded Merganser. On one occasion, we observed a Whistling-Duck pair incubating a nest containing Whistling-Duck, Wood Duck, and Hooded Merganser eggs. Although the Wood Duck eggs were lost during incubation because of a suspected partial depredation or removal by the pair, two Hooded Merganser eggs were successfully hatched along with 16 Whistling-Duck ducklings. IBP did not lower hatchability, as mixed and normal clutches hatched similar percentages of eggs. Our results suggest IBP via Wood Duck and Hooded Merganser likely does not limit Whistling-Duck productivity; however, the implications of CBP are less clear.
We found CBP present in ~43% of the clutches we observed, whereas previous studies have estimated CBP in 70–100% of Whistling-Duck nests (Delnicki 1973, McCamant and Bolen 1979, James 2000). However, our analysis of egg morphology showed low estimates of repeatability, suggesting that CBP was likely present in some normal and mixed clutches. Thus, classifying CBP clutches using the techniques described in our methods likely underestimates the number of nests with CBP present. As a result, the true prevalence of CBP in Whistling-Ducks remains somewhat unclear, and future research should consider using genetic techniques to unequivocally quantify rates of parasitism (Pöysä et al. 2009, Eadie et al. 2010, Lemons et al. 2011).
CBP and mixed clutches were generally larger than normal clutches. The number of eggs in mixed clutches closely resembled CBP clutches, except in April when they were smaller. Many Wood Ducks are still incubating their first nest attempts in April, potentially limiting IBP opportunity, whereas later in the season, renesting Wood Ducks and Whistling-Ducks attempting their first nests will simultaneously lay eggs in vacant nest boxes, thus facilitating mixed clutches. Although we had expected parasitism to reduce hatchability, both CPB and mixed clutches had higher hatchability than normal clutches. Thus, Whistling-Ducks may actually benefit from parasitism, and ~75% of Whistling-Duck ducklings hatched from parasitized nests (Sorenson 1991, Péron and Koons 2012).
In general, we found the percentage of nests incubated (48.3%), and the hatchability of both successful (64.7%) and all nests (30.0%) were comparable to previous studies (Bolen 1967, McCamant and Bolen 1979, O'Kelley 1987). Although other studies have documented that large Whistling-Duck and Wood Duck clutches have lower hatchability (O’Kelley 1987, Semel et al. 1988), we found no such pattern. Considering only those nests that hatched at least one egg, hatchability was reliably > 60% for all clutch sizes of 11–45 eggs. Whistling-Ducks incubating large clutches routinely hatched 20–30 eggs, which further suggests that the costs of parasitism are relatively low in this system.
The baseline breeding ecology data provided here is an important step in filling the gaps in our knowledge about this under-studied species. Although nest box programs could be facilitating Whistling-Duck range expansion, further research to quantify productivity of other nesting strategies (e.g., natural cavity and ground nests) is needed. Additionally, our observations in the field often left us with more questions than answers. For example, upon arrival in spring, Whistling-Ducks appeared to remain in family groups; it is unknown whether the high rates of CBP we observed in this study are instances of kin laying in the same box (Andersson et al. 2019), which, combined with astonishingly high rates of hatchability for large clutches, could dramatically increase inclusive fitness. Additionally, although we documented many instances of mixed broods hatched by Whistling-Ducks, we (anecdotally) never observed mixed broods of ducklings led by Whistling-Ducks in the field (D. L. Bakner and K. M. Ringelman, Louisiana State University, personal observation). It is unclear whether species re-assort post-hatch, or whether Wood Duck ducklings are driven off, killed, or otherwise suffer differential mortality. However, in 2021, we recaptured two nesting Wood Duck hens that hatched in 2020 as part of a single mixed clutch incubated by Whistling-Ducks (D. L. Bakner and K. M. Ringelman, unpublished data). Clearly, there are bountiful opportunities for further investigation of Whistling-Duck ecology, and how they compete with (or facilitate) species like Wood Ducks and Hooded Mergansers remains an important research priority as Whistling-Ducks continue to expand their range northward.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.ACKNOWLEDGMENTS
Funding for this project was provided by the Louisiana Department of Wildlife and Fisheries, United States Department of Agriculture; National Institute of Food and Agriculture McIntire-Stennis grant LAB94294, Louisiana State University College of Agriculture, Louisiana State University Agricultural Center, United Waterfowlers of Florida, and the Louisiana Ornithological Society. We thank N. Bosco, J. Dubman, C. Jones, N. Ragucci, C. Tiemann, T. Vidrine, and A. Yaw for helping collect field data. KMR acquired the majority of project funding with additional grants to DLB and KEM; DLB and KEM collected the data; KMR and DLB conceived the analyses, and DLB and KEM conducted them; DLB and KEM wrote the paper with edits from KMR. We conducted our work under U.S. Fish and Wildlife Service banding permit #06669 and Special Use Permit 43614-20-04; Louisiana Department of Wildlife and Fisheries state collecting permits WDP-20-037 and WDP-21-060, and Wildlife Management Area Permit WL-Research-2020-03; Louisiana State University Institutional Animal Care and Use Protocol A2019-27.
DATA AVAILABILITY
The datasets to reproduce our analyses are available on Dryad https://doi.org/10.5061/dryad.dv41ns22m
LITERATURE CITED
Andersson, M., M. Åhlund, and P. Waldeck. 2019. Brood parasitism, relatedness and sociality: a kinship role in female reproductive tactics. Biological Reviews 94:307-327. https://doi.org/10.1111/brv.12455
Baldassarre, G. 2014. Ducks, geese, and swans of North America. Johns Hopkins University Press, Baltimore, Maryland, USA.
Bergstrom, B. J. 1999. First reported breeding of Black-bellied Whistling-Duck in Northern Florida. Florida Field Naturalist 27:177-179.
Bolen, E. G. 1967. The ecology of the Black-bellied Tree Duck in Southern Texas. Dissertation. Utah State University, Logan, Utah, USA.
Bolen, E. G., and B. W. Cain. 1968. Mixed Wood Duck-Tree Duck clutch in Texas. Condor 70:389-390. https://doi.org/10.2307/1365938
Bolen, E. G., B. McDaniel, and C. Cottam. 1964. Natural history of the Black-bellied Tree Duck (Dendrocygna autumnalis) in Southern Texas. Southwestern Naturalist 9:78-88. https://doi.org/10.2307/3668787
Bourne, G. R. 1981. Food habits of Black-bellied Whistling Ducks occupying rice culture habitats. Wilson Bulletin 93:551-554.
Cohen, B. S., S. E. Askin, G. D. Balkcom, R. J. Benedict, Jr., J. A. Rader, J. D. James, B. A. Collier, and M. J. Chamberlain. 2019. Survival and distribution of Black-bellied Whistling-Duck (Dendrocygna autumnalis) in the Southeastern United States. Journal of the Southeastern Association of Fish and Wildlife Agencies 6:123-128.
Croft, G. D., R. M. Kaminski, E. P. Wiggers, P. D. Gerard, and G. K. Yarrow. 2020. Nest-box use by Wood Ducks and Black-bellied Whistling Ducks in coastal South Carolina. Wildlife Society Bulletin 44:662-669. https://doi.org/10.1002/wsb.1135
Delnicki, D. E. 1973. Renesting, incubation behavior, and compound clutches of the Black-bellied Tree Duck in Southern Texas. Thesis. Texas Tech University, Lubbock, Texas, USA.
DuRant, S. E., G. R. Hepp, I. T. Moore, B. C. Hopkins, and W. A. Hopkins. 2010. Slight differences in incubation temperature affect early growth and stress endocrinology of Wood Duck (Aix sponsa) ducklings. Journal of Experimental Biology 213:45-51. https://doi.org/10.1242/jeb.034488
Eadie, J. M., J. N. M. Smith, D. Zadworny, U. Kühnlein, and K. Cheng. 2010. Probing parentage in parasitic birds: an evaluation of methods to detect conspecific brood parasitism using Goldeneyes Bucephala islandica and Bl. clangula as a test case. Journal of Avian Biology 41:163-176. https://doi.org/10.1111/j.1600-048X.2009.04735.x
Edmonds, S. T., and D. S. Stolley. 2008. Population decline of ground-nesting Black-bellied Whistling Ducks (Dendrocygna autumnalis) on islands in Southern Texas. Southwestern Naturalist 53:185-189. https://doi.org/10.1894/0038-4909(2008)53[185:PDOGBW]2.0.CO;2
Emery, R. B., D. W. Howerter, L. M. Armstrong, M. G. Anderson, J. H. Devries, and B. L. Joynt. 2005. Seasonal variation in waterfowl nesting success and its relation to cover management in the Canadian Prairies. Journal of Wildlife Management 69:1181-1193. https://doi.org/10.2193/0022-541X(2005)069[1181:SVIWNS]2.0.CO;2
Harper, D. G. C. 1994. Some comments on the repeatability of measurements. Ringing and Migration 15:84-90. https://doi.org/10.1080/03078698.1994.9674078
Hepp, G. R., R. A. Kennamer, and M. H. Johnson. 2006. Maternal effects in Wood Ducks: incubation temperature influences incubation period and neonate phenotype. Functional Ecology 20:308-314. https://doi.org/10.1111/j.1365-2435.2006.01108.x
James, J. D. 2000. Effects of habitat and spatial characteristics on the incidence of conspecific brood parasitism and nest site selection in breeding Black-bellied Whistling Ducks. Thesis. Texas A&M University, Kingsville, Texas, USA.
James, J. D., and J. E. Thompson. 2020. Black-bellied Whistling-Duck (Dendrocygna autumnalis), version 1.0. In A. F. Poole, and F. B. Gill, editors. Birds of the world, Cornell Lab of Ornithology, Ithaca, New York, USA. https://doi.org/https://doi.org/10.2173/bow.bbwduc.01
Lemons, P. R., J. S. Sedinger, and P. S. Randle. 2011. Detecting conspecific brood parasitism using egg morphology in Black Brant Branta bernicla nigricans. Journal of Avian Biology 42:282-288. https://doi.org/10.1111/j.1600-048X.2011.05217.x
Lessells, C. M., and P. T. Boag. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104:116-121. https://doi.org/10.2307/4087240
MacWhirter, R. B. 1989. Minireview: on the rarity of intraspecific brood parasitism. Condor 91:485-492. https://doi.org/10.2307/1368333
Mallory, M. L., and P. J. Weatherhead. 1990. Effects of nest parasitism and nest location on eggshell strength in waterfowl. Condor 92:1031-1039. https://doi.org/10.2307/1368739
Markum, D. E., and G. A. Baldassarre. 1989. Ground nesting by Black-bellied Whistling Ducks on islands in Mexico. Journal of Wildlife Management 53:707-713. https://doi.org/10.2307/3809201
Matta, N. E., M. A. Pacheco, A. A. Escalante, G. Valkiūnas, F. Ayerbe-Quiñones, and L. D. Acevedo-Cendales. 2014. Description and molecular characterization of Haemoproteus macrovacuolatus n. sp.(Haemosporida, Haemoproteidae), a morphologically unique blood parasite of Black-bellied Whistling Duck (Dendrocygna autumnalis) from South America. Parasitology Research 113:2991-3000. https://doi.org/10.1007/s00436-014-3961-2
McCamant, R. E., and E. G. Bolen. 1979. A 12-year study of nest box utilization by Black-bellied Whistling Ducks. Journal of Wildlife Management 43:936-943. https://doi.org/10.2307/3808277
O’Kelley, B. L. 1987. Recruitment of Black-bellied Whistling-Ducks in South Texas with special reference to the use of nest boxes. Thesis. Texas Tech University, Lubbock, Texas, USA.
Péron, G., and D. N. Koons. 2012. Integrated modeling of communities: parasitism, competition, and demographic synchrony in sympatric ducks. Ecology 93:2456-2464. https://doi.org/10.1890/11-1881.1
Pöysä, H., K. Lindblom, J. Rutila, and J. Sorjonen. 2009. Reliability of egg morphology to detect conspecific brood parasitism in Goldeneyes Bucephala clangula examined using protein fingerprinting. Journal of Avian Biology 40:453-456. https://doi.org/10.1111/j.1600-048X.2008.04528.x
R Core Team. 2021. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Semel, B., P. W. Sherman, and S. M. Byers. 1988. Effects of brood parasitism and nest-box placement on Wood Duck breeding ecology. Condor 90:920-930. https://doi.org/10.2307/1368849
Sorenson, M. D. 1991. The functional significance of parasitic egg laying and typical nesting in redhead ducks: an analysis of individual behaviour. Animal Behaviour 42:771-796. https://doi.org/10.1016/S0003-3472(05)80122-8
Weller, M. W. 1956. A simple field candler for waterfowl eggs. Journal of Wildlife Management 20:111-113. https://doi.org/10.2307/3797414
Fig. 1
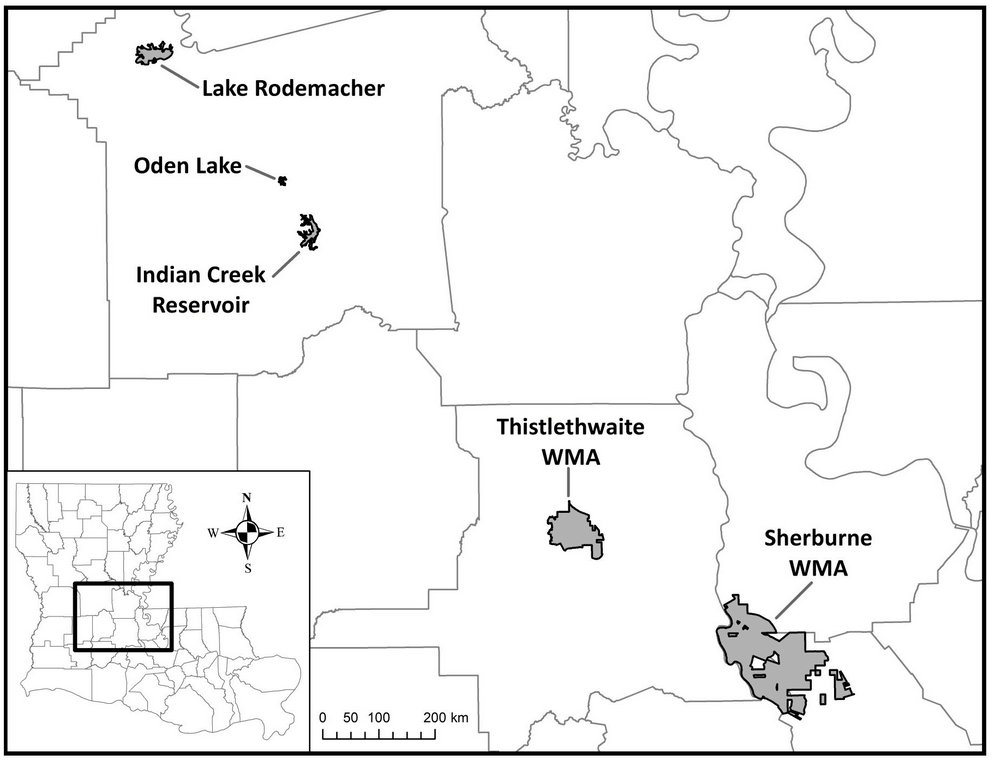
Fig. 1. Map of Louisiana with study areas outlined in gray. Lake Rodemacher, Oden Lake, and Indian Creek Reservoir are located in Rapides Parish, whereas Thistlethwaite Wildlife Management Area is located in St. Landry Parish. Sherburne Wildlife Management Area is located within Iberville, St. Martin, and Point Coupee Parishes.

Fig. 2
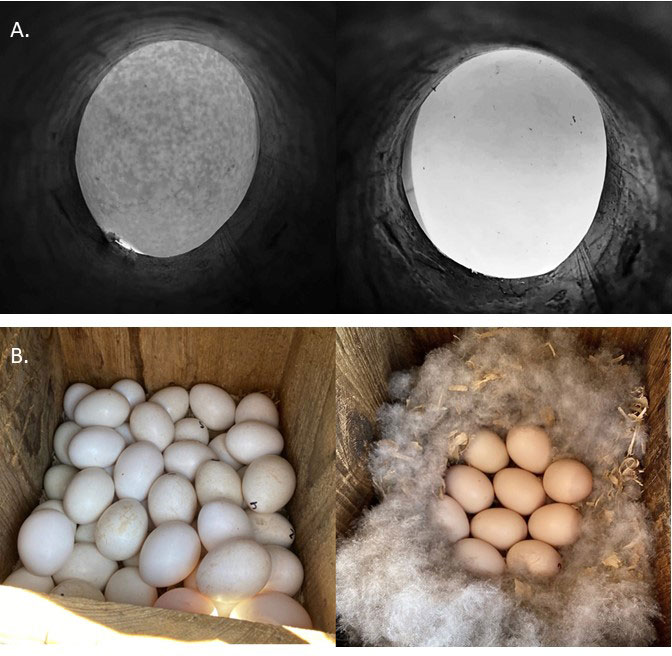
Fig. 2. (A) Comparison of Black-bellied Whistling-Duck (Dendrocygna autumnalis; [Whistling-Duck]; left) and Wood Duck (Aix sponsa; right) eggshell transparency. Note the distinct mottled pattern of the Whistling-Duck egg, which becomes less visible as incubation progresses. (B) Comparison of Whistling-Duck (left) and Wood Duck (right) eggs as they appear in the nest box. Note that Whistling-Duck eggs are bright white and nests do not typically have down feathers present.

Fig. 3
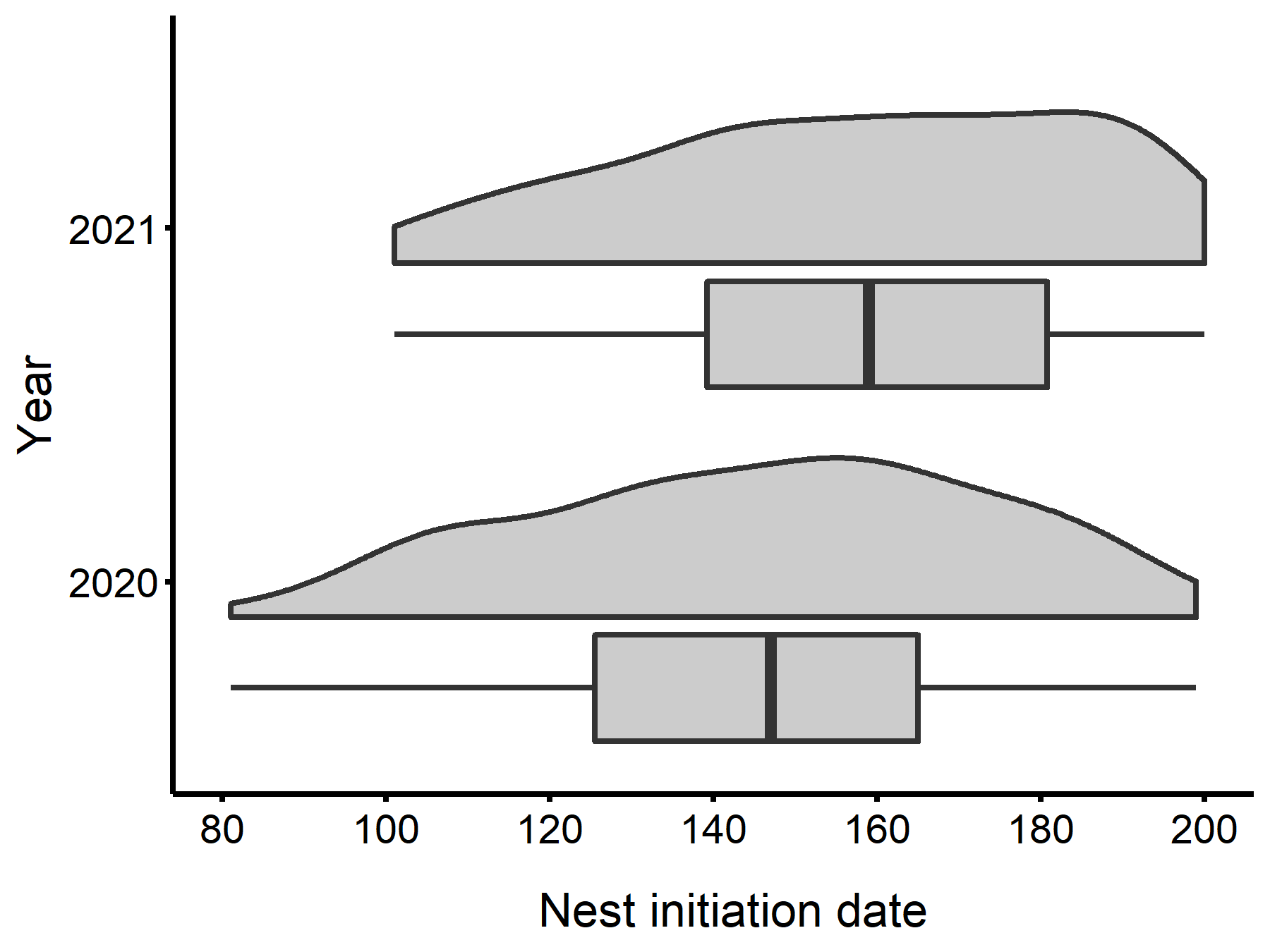
Fig. 3. Julian dates for initiated Black-bellied Whistling-Duck (Dendrocygna autumnalis) nests in Louisiana (n = 126 and 135 nests in 2020 and 2021, respectively). The distributions above each boxplot are density plots drawn from the observed data points.

Fig. 4
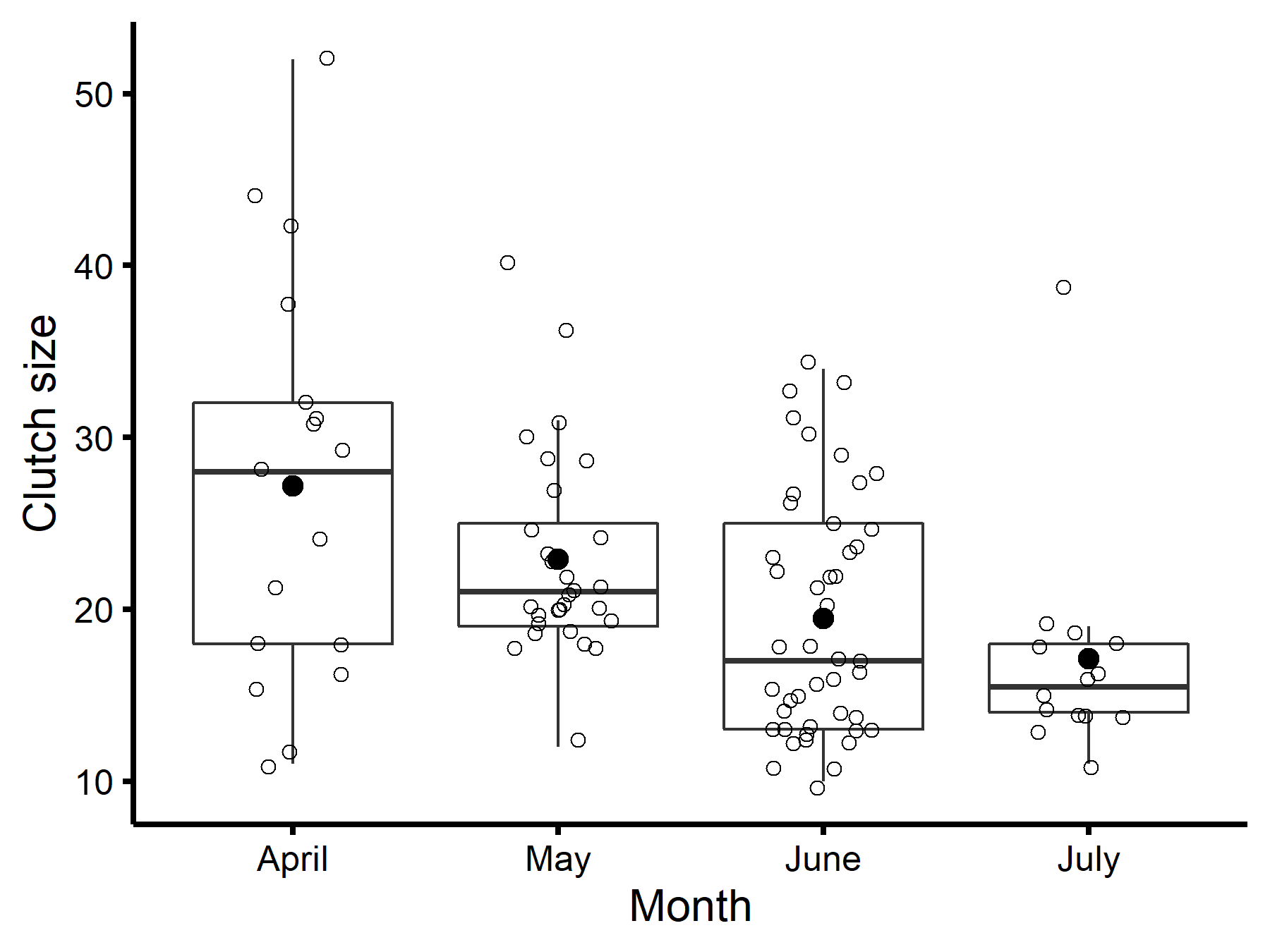
Fig. 4. Clutch size of Black-bellied Whistling-Duck (Dendrocygna autumnalis) nests in Louisiana by month with the means dotted in black and the center line of each boxplot representing medians.

Table 1
Table 1. Monthly sample sizes and summary statistics of Black-bellied Whistling-Duck (Dendrocygna autumnalis; Whistling-Duck) clutches in Louisiana during 2020 and 2021. Normal clutches contained only Whistling-Duck eggs, conspecific brood parasitism (CBP) clutches received > 1 egg per day during the laying stage or additional eggs following day four of incubation, and mixed clutches contained ≥ 1 Wood Duck (Aix sponsa) or Hooded Merganser (Lophodytes cucullatus) egg.
| Incubated clutches (n) | Clutch size ( ± SD) | ||||||||
| Month | Initiated | Incubated† | Normal | CBP | Mixed | Normal | CBP | Mixed | |
| March | 2 | 0 | 0 | 0 | 0 | ||||
| April | 38 | 17 | 2 | 8 | 7 | 21.5 ± 14.8 | 34.6 ± 11.4 | 20.3 ± 7.2 | |
| May | 79 | 29 | 5 | 13 | 11 | 18.2 ± 3.5 | 24.5 ± 7.3 | 23.9 ± 4.3 | |
| June | 87 | 45 | 19 | 21 | 5 | 14.1 ± 3.6 | 23.6 ± 6.3 | 24.9 ± 5.8 | |
| July | 55 | 14† | 12 | 2 | 0 | 15.2 ± 2.45 | 28.5 ± 14.8 | ||
| Total | 261 | 105 | 38 | 44 | 23 | ||||
| † Does not include nests with incubation starting after 31 July. | |||||||||
Table 2
Table 2. Mean square variation among (MSA) and within clutches (MSW), F-statistics, p-values, and repeatability (r) estimates for Black-bellied Whistling-Duck (Dendrocygna autumnalis) eggs measured from nests where conspecific brood parasitism (CBP) was present and absent. The coefficient value related to sample size per group (n0) was used to calculate repeatability (Lessells and Boag 1987).
| Measurement | MSA | MSW | F-statistic | p-value | r |
| CBP clutches (n = 44 nests, 1108 eggs, n0 = 25.66) | |||||
| Egg length | 20.65 | 3.54 | 5.83 | < 0.0001 | 0.16 |
| Egg width | 8.04 | 1.12 | 7.17 | < 0.0001 | 0.19 |
| Egg mass | 77.67 | 10.90 | 7.13 | < 0.0001 | 0.19 |
| Normal clutches (n = 21 nests, 241 eggs, n0 = 11.81) | |||||
| Egg length | 15.39 | 2.61 | 5.91 | < 0.0001 | 0.29 |
| Egg width | 4.75 | 0.80 | 5.91 | < 0.0001 | 0.29 |
| Egg mass | 57.12 | 8.01 | 7.13 | < 0.0001 | 0.34 |
Table 3
Table 3. Total number of Louisiana Black-bellied Whistling-Duck (Dendrocygna autumnalis) nests and hatchability of eggs across clutch size bins.
| Hatchability | |||||
| Clutch size | Nests | Eggs laid | Eggs hatched | All nests | Successful nests |
| 1–5 | 29 | 67 | 0 | 0.0% | |
| 6–10 | 8 | 68 | 0 | 0.0% | |
| 11–15 | 27 | 357 | 112 | 31.4% | 81.6% |
| 16–20 | 37 | 663 | 228 | 34.4% | 73.3% |
| 21–25 | 33 | 754 | 209 | 27.7% | 60.0% |
| 26–30 | 20 | 555 | 165 | 29.7% | 65.2% |
| 31–35 | 13 | 422 | 192 | 45.5% | 66.9% |
| 36–40 | 3 | 114 | 49 | 43.0% | 62.8% |
| 41–45 | 7 | 298 | 58 | 19.5% | 67.4% |
| 46–50 | 0 | ||||
| 51–55 | 3 | 158 | 25 | 15.8% | 24.3% |
| Total | 180 | 3456 | 1038 | 30.0% | 64.7% |
Table 4
Table 4. Total number of Louisiana Black-bellied Whistling-Duck (Dendrocygna autumnalis; Whistling-Duck) nests and hatchability of eggs by month from 2020–2021. Normal clutches contained only Whistling-Duck eggs, conspecific brood parasitism (CBP) clutches received greater than one egg per day during the laying stage or additional eggs following day four of incubation, and mixed clutches contained greater than one Wood Duck (Aix sponsa) or Hooded Merganser (Lophodytes cucullatus) egg.
| Clutch Type | March | April | May | June | Total† | ||
| Normal | |||||||
| Nests | 0 | 7 | 19 | 34 | 60 | ||
| Eggs laid | 0 | 77 | 211 | 271 | 559 | ||
| Eggs hatched | 0 | 26 | 37 | 86 | 149 | ||
| Hatchability | NA | 33.8% | 17.5% | 31.7% | 26.7% | ||
| CBP | |||||||
| Nests | 0 | 13 | 25 | 20 | 58 | ||
| Eggs laid | 0 | 440 | 595 | 453 | 1,488 | ||
| Eggs hatched | 0 | 190 | 154 | 163 | 507 | ||
| Hatchability | NA | 43.2% | 25.9% | 36.0% | 34.1% | ||
| Mixed | |||||||
| Nests | 2 | 10 | 28 | 6 | 46 | ||
| Eggs laid | 32 | 214 | 688 | 157 | 1,091 | ||
| Eggs hatched | 0 | 46 | 138 | 94 | 278 | ||
| Hatchability | 0.00% | 21.5% | 20.1% | 59.9% | 25.5% | ||









