The following is the established format for referencing this article:
Zhou, A., Y. Wang, and Y. Chen. 2022. Diet composition based on stable isotopic analysis of fecal samples reveals the preference of Black-faced Spoonbill (Platalea minor) for natural wetlands and fishponds. Journal of Field Ornithology 93(3):7.ABSTRACT
The Black-faced Spoonbill (Platalea minor; BFS) is a globally endangered species that is distributed only in the coastal zones of East Asia. Xinghua Bay is one of the main wintering sites and migration stopovers of BFS in mainland China. However, with the reduction and degradation of natural wetlands, it is uncertain whether artificial wetlands can provide habitat for the endangered BFS. Research on the diet of BFS will help to understand their preferences between natural and artificial wetlands and provide a reference for their conservation and habitat restoration. From December 2017 to February 2020, 45 potential food samples and 199 fecal samples of BFS were collected during six sampling periods, of which Cyprinidae, Mugilidae, Portunidae, Gobiidae and Palaemonidae were collected from natural wetlands and Crucian (Carassius auratus) and whiteleg shrimp (Litopenaeus vannamei) were collected from fishponds. Their stable isotope values (e.g., δ13C and δ15N) were measured to obtain the food composition information for BFS. In early winter, the proportion of Palaemonidae in BFS food was as high as 74.4%, while those of other foods were only 3.0% to 6.0%. In late winter, the food contributions for BFS were as follows: Portunidae 39.3% > Palaemonidae 26.1% > Cyprinidae 8.8% > Mugilidae 8.5% > Gobiidae 7.3% > Crucian 5.1% > whiteleg shrimp 4.8%. The proportion of Portunidae exceeded that of Palaemonidae, and together with Palaemonidae, it became the main food of BFS in late winter. The diet compositions of BFS between early and late winter were significantly different, which may be due to seasonal changes in food resources. Natural wetlands are the main feeding grounds of BFS, but fishponds also provide them with supplementary feeding grounds and resting places. Fishponds play an important ecological function in maintaining the overwintering population of BFS in Xinghua Bay.RESUMEN
INTRODUCTION
The Black-faced Spoonbill (Platalea minor; BFS) is an endangered waterbird belonging to Ciconiiformes and Threskiorothidae (Jin et al. 2009). Because the population decreased far lower than the historical number (Pickett et al. 2018), with an increase in protection, the BFS population has been increasing in recent years, i.e., changed from less than 1000 individuals in 2002 to 5222 individuals in 2021 (Yu et al. 2021). However, BFS is still listed as endangered, which is largely due to its narrow distribution area and threats from human living activities. BFS are distributed only in the coastal zones of East Asia and forage and rest only in intertidal zones or within 2-3 km of tidal areas. However, the high-intensity development of coastal zones in recent years has greatly reduced the area or quality of coastal wetlands (Yu and Swennen 2004). According to Pickett et al.’s prediction, climate change and habitat loss together threaten the recovery of the BFS population such that, by 2050, population declines of BFS might be apparent as a consequence of these cumulative impacts (Pickett et al. 2018).
It is a global phenomenon that natural wetlands are being transformed into artificial wetlands, such as rice fields, artificial ponds, or reservoirs (Li et al. 2013). Ornithologists have conducted much research on whether artificial wetlands can replace natural wetlands to provide suitable habitats for waterbirds. Some studies have shown that well-protected natural wetlands provide more suitable habitats for waterbirds than artificial wetlands with more species and higher densities of waterbirds (Ma et al. 2004, Li et al. 2013, Lou et al. 2019). Although other studies have shown that artificial wetlands can provide alternative habitats for the breeding, wintering, and migration of waterbirds (Masero 2003, Rajpar and Zakaria 2013), newly built or poorly managed artificial wetlands cannot maintain stable biological communities because of a lack of stable food resources (Choi et al. 2014). The BFS makes use of artificial wetlands as resting grounds and opportunistically chooses natural and artificial wetlands for foraging (Swennen and Yu 2005), but their preference for using these two types of wetlands is not clear. It is difficult to directly determine the foraging habitat of BFS by conventional observation methods because they mainly forage at dawn, dusk, and night and rest most of the day (Ruan et al. 2006). The differences in bird utilization of different habitats in the same geographical area are the result of the comprehensive effect of many environmental factors, and food resources are the decisive factors affecting the habitat utilization mechanism of birds (Luo et al. 2013). Therefore, based on the stable isotope technique, we used fecal samples to study the food compositions of BFS and attempted to determine their preference for natural wetlands and fishponds, as well as the survival status of BFS in Xinghua Bay.
In the past, most studies on the dietary compositions of birds have mainly used stomach content analysis (Lilliendahl and Solmundsson 2006) and fecal microscopic analysis (Gasperin and Pizo 2009). However, both methods have some limitations, which may overestimate the indigestible resources. Moreover, collecting stomach contents may harm individuals and is not suitable for rare and endangered birds. For fecal microscopic examinations, the labor and time costs are high, and the accuracy of food determinations is very low because of the limitations of debris identification (Nielsen et al. 2018). By measuring the stable isotope values in bird tissues or feces and in potential food sources and establishing a linear mixed model (such as a Bayesian model) between them, the food composition of birds can be obtained through the model. The stable isotopes commonly used in diet research consist of carbon (e.g., 13C/12C) and nitrogen (e.g., 15N/14N). The fractionation effect of stable carbon isotopes is not obvious, and the discrimination factor is small, which makes it an ideal element for studying the food compositions of animals. Stable nitrogen isotopes have stable and large trophic grade discrimination factors between food and their predator tissues, so they are often used for analyzing food composition and trophic structures (Boecklen et al. 2011, Wang et al. 2015). Generally, the tissues used in the study of bird diets by applying the stable isotope technique include blood, muscle, and feathers (Inger and Bearhop 2008, Wang et al. 2015). As BFS are endangered, obtaining blood and muscle samples may cause great harm to individual birds, and feces are the most nondestructive samples that reflect bird diets. Moreover, the sample size of feces accumulates rapidly compared with blood, muscle, or other tissues.
Ueng et al. analyzed the stomach contents of more than 40 BFS that were poisoned in Chinese Taiwan (Ueng et al. 2006). This result showed that fish and shrimp were the main foods of BFS, which was confirmed by studies in other distribution areas, such as the coasts of the Chinese mainland, Hong Kong, South Korea, Japan, and Vietnam (Swennen and Yu 2005, Takano et al. 2014, Huang et al. 2021). However, their research did not show the relative proportions of the food compositions of BFS, nor could they show the relative utilization preference of BFS for natural and artificial wetlands. Jeong et al. compared the stable isotope values at the beginning and end of broiler feathers of BFS breeding at offshore sites in South Korea and found that the food of chicks mainly came from freshwater environments, and the proportion of freshwater food in the early stage was higher than that in the later stage (Jeong et al. 2021). The food composition of the BFS varies with different habitat types and distribution areas. Xinghua Bay in Fujian Province is an important migration stopover site and wintering ground for BFS where 238 individuals were recorded in January 2021, exceeding 20% of the overwintering population in mainland China (Yu et al. 2021). For this research, we collected feces and potential food samples of BFS in Xinghua Bay through three winters and analyzed the stable isotope (e.g., δ13C and δ15N) values of samples to study the relative preference of BFS for natural wetlands and fishponds.
METHODS
Study site
Xinghua Bay is located on the middle coast of Fujian Province (25°14′-25°37′N, 119°00′-119°37′E). It is the largest semi-enclosed bay in Fujian Province, covering an area of approximately 620 km² (Li et al. 1999; Fig. 1). It contains a high diversity of waterbirds because of its large area of shallow-water wetlands and rich food resources. In a single survey conducted in Xinghua Bay, the largest wintering population of waterbirds reached 31,275 (Wang and Zhang 2012). The populations of Saunders’s Gull (Saundersilarus saundersi), BFS, Kentish Plover (Charadrius alexandrinus), and Eurasian Curlew (Numenius arquata) accounted for more than 1% of the global population (Zhang et al. 2019), which met the standards for internationally important wetlands (Xia et al. 2017). Therefore, the establishment of a provincial waterbird nature reserve in Xinghua Bay was approved in January 2022. Large numbers of waterbirds overwinter in Xinghua Bay and use not only natural wetlands but also the large areas of aquaculture ponds that have been operated in this area for a long time.
Sample collection
The BFS arrives at Xinghua Bay in early November and leaves at the end of March of the following year. Among them, after omitting the autumn and spring migrations, we divided the overwintering period into early (e.g., November to December) and late parts (e.g., January to February). Samples were collected six times from December 2017 to February 2021, including 45 potential food samples and 199 fecal samples. The potential food samples were purchased from local fishermen, and their fishing locations were specified to distinguish whether the food samples came from natural wetlands or fishponds. When collecting fecal samples, we observed resting BFS groups for half an hour and then approached and carefully scraped the feces with plastic sheets and placed them into 5-ml centrifuge tubes. To avoid pollution by impurities such as soil, we scraped only the middle parts of the feces. BFS often mingle with other waterbirds to rest on the ridges of aquaculture ponds, mainly with Gray Herons (Ardea cinerea). Unlike other waterbirds, BFS like to gather as a single species, while herons spread and rest at equal distances to bask in the sun. Moreover, the feces of these two species look very different. The feces of BFS are relatively small, and there are solid blocks in the middle of a white liquid, whereas the feces of herons are formed in the shape of large pools of liquid jets. Therefore, as long as we fully understand the relative positions and fecal differences, we can ensure that the collected fecal samples belonged to BFS. All samples were stored in a freezer at -20 °C.
Stable isotopic analysis (SIA)
After rinsing with distilled water, the food samples were marked and photographed. Then, we measured their lengths, widths, and weights. The photos were given to experts for species identification. When taking thumb-sized muscle samples, the back muscle was taken from fish, the abdominal muscle was taken from shrimp, and the leg muscle was taken from crabs. The samples were then soaked in 1 mol/L HCl for 2 hours to remove inorganic carbon from the sample surfaces, rinsed again with distilled water, and dried in an oven at 60 °C for 12 hours. Because the degreasing procedure may affect the true nitrogen isotope values of samples, it is necessary to divide the samples into two categories: one category uses direct measurements, the δ15N stable isotope values, and the other measures these values after degreasing. Degreasing was performed by soaking the samples in 0.25 mol/L NaOH solution for 2 hours (Li et al. 2016, Wang et al. 2017).
After being powdered, the samples were analyzed using a stable isotope ratio mass spectrometer (EA-IRMS) at the Stable Isotope Center at Fujian Agriculture and Forestry University, and the instrument manufacturers were Elementar (Germany) and Isoprime (UK). The formula for calculating the stable carbon and nitrogen isotope values is as follows:
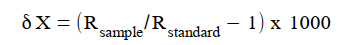 |
(1) |
where X refers to 13C or 15N, Rsample refers to the stable isotope ratios, 13C/12C or 15N/14N, of the measured samples, Rstandard refers to the stable isotope ratio, 13C/12C or 15N/14N, of the international standard substance. The 13C/12C and 15N/14N ratios are reported relative to the Vienna PeeDee Belemnite and atmospheric N2 standards, respectively.
Data analysis
The means and standard deviations of the stable isotope values of samples were calculated, and the outliers that deviated from the mean by more than twice the standard deviation were screened out. A total of 14 potential food sources were divided into 7 groups, of which Cyprinidae, Mugilidae, Portunidae, Gobiidae, and Palaemonidae were collected from natural wetlands and Crucian and whiteleg shrimp were collected from fishponds.
The differences in δ13C and δ15N values between fecal samples and potential food samples were compared by one-way ANOVA. All isotope data are expressed as the mean ± standard deviation (mean ± SE), and p < 0.05 was considered significant.
The most commonly used model to study food compositions by stable isotope analysis is the Bayesian mixing model. It can consider the variable nondeterministic factors with relatively high accuracy and has a broad range of application. We used the R package simmr, which can be used to process the mixing equations of stable isotope data within a Bayesian mixing model framework (Parnell et al. 2010, 2013). The fecal values were calibrated by the discrimination factors (δ13C = 1.15‰ and δ15N = 2.91‰), which were estimated for fecal samples of birds in general (Caut et al. 2009).
RESULTS
Stable isotope characteristics of BFS feces and potential food samples
The δ13C values of BFS feces were mainly distributed within the ranges of the 7 groups of potential foods, which showed that the collected potential foods can represent the diet composition of BFS overwintering in Xinghua Bay (Fig. 2). The δ13C values of the 7 potential foods ranged from -26.55‰ to -17.26‰ with very significant differences (F6, 19 = 5.98, p < 0.01), while the δ15N values ranged from 7.92‰ to 13.98‰ with significant differences (F6,19 = 3.34, p < 0.05). The δ13C values of the potential foods from natural wetlands and fishponds separately ranged from -25.28‰ to -15.95‰ and -26.55‰ to -26.20‰, respectively, while the δ15N values ranged from 7.92‰ to 15.41‰ and 13.30‰ to 13.98‰, respectively. There were no significant differences in δ15N values (F1,24 = 9.56, p > 0.05) but there was a significant difference in δ13C values (F1,24 = 9.56, p < 0.01) between natural wetlands and fishponds. Therefore, δ13C and δ15N may be combined to indicate the feeding preferences of BFS. The differences in fecal values between early and late winter were significant (δ13C: F1,46=20.49, p < 0.001, δ15N: F1,46=71.25, p < 0.001). The fecal δ13C values were concentrated in the range of the δ13C values of Cyprinidae, Mugilidae, and Palaemonidae in early winter, while they were concentrated in the range of the δ13C values of Mugilidae, Portunidae, and Gobiidae in late winter (Table 1), which means that BFS may have transformed their food habits throughout the overwintering period.
Diet composition inferred by SIA
The stable isotope analysis showed that in early winter, Palaemonidae contributed the most to the diet of BFS, with 74.4%, while the contribution rates of the other foods were smaller, ranging from 3.0% to 6.0% (Fig. 3). In late winter, the contribution rates of the various foods were as follows: Portunidae (39.3%) > Palaemonidae (26.1%) > Cyprinidae (8.8%) > Mugilidae (8.5%) > Gobiidae (7.3%) > Crucian (5.1%) > whiteleg shrimp (4.8%; Table 2, Fig. 4). The combined contribution rate of Portunidae and Palaemonidae reached 65.4% and became the most important food of BFS in late winter. The contribution rates of foods coming from natural wetlands were 93.4% in early winter and 90.0% in late winter, while those collected from fishponds were 6.0% in early winter and 9.9% in late winter. These results showed that BFS mainly used natural wetlands for foraging grounds while overwintering in Xinghua Bay, whereas fishponds could also provide a small part of the food supply.
DISCUSSION
The food composition of BFS overwintering in Xinghua Bay
Jin et al. (2010) investigated the potential foods of BFS in the coastal wetlands of Xinghua Bay from 2007 to 2008 and estimated that their potential foods might include 19 species of fish and 6 species of shrimp. Among these potential foods, Mugilidae and Gobiidae were the most abundant (Jin et al. 2010). In our results, fishes represent only a small proportion among the foods of BFS, which indicated that it is not adequate to estimate the diet of BFS by only using the species of potential foods because BFS may have some foraging strategies and habits that we are not aware of. In addition, the fecal samples used in the SIA contain indigestible parts such as bone and scale of fishes and shell of shrimp and crabs. However, only the muscle in the potential preys were extracted. Fractionation of stable isotopes result in small differences between the muscle values and the hard part values, which will impact the proportion of potential prey. Our results might underestimate the contribution of fishes with low rate of hard part of body material.
The low proportion of fishes might be due to the reduction of fish resources in the coastal wetlands of Xinghua Bay, while the reduction of shrimp and crabs were relatively small. Over the past decade, coastal development of Xinghua Bay has increased, and many projects have been or are under construction, such as ports, wind farms, and nuclear power plants (Wu et al. 2019). Agricultural, domestic, and industrial pollution are continuously decreasing the quality of wetlands because of their weak self-purification ability (Liu 2006). Eutrophication of the Xinghua Bay coastal wetlands is very serious, and both the inorganic nitrogen and organic phosphorus concentrations exceed the standard (Zhong et al. 2011). With the continuous deterioration of wetland quality and long-term high-intensity fishing, the fishery resources along the coasts of Fujian Province are significantly depleted (Huang et al. 2010, Luo 2018). In addition, dams built on rivers in Fujian Province have affected the breeding activities of migratory fishes, such as Gobiidae (Li et al. 2007), which might also be one of the reasons for the decline in fish sources. Meanwhile, the shrimp and crabs were not affected by the dams so their reduction of number might be relatively small. The coastal animal resources in Xinghua Bay need to be investigated.
Our results showed that the food composition of BFS has significant differences between early and late winter. In early winter, Palaemonidae was the only main food, with a contribution rate of 74.4%. In late winter, the proportion of Palaemonidae decreased to 26.1%, while that of Portunidae increased to 39.3% from 6.0% and that of fishes increased to 29.7% from 16.0%. BFS is an opportunistic predator (Swennen and Yu 2005). The change in overwintering diets probably reflects the change in relative abundances of food resources. Some studies have shown that the biomass of crabs in intertidal zones increases gradually from summer to winter and reaches a peak in winter (Chen and Xu 1992). On the other hand, the shrimp in the intertidal zone may migrate to deep-water areas in late winter to avoid low temperatures (Xu and Sun 2013). With the progression of winter, the number of shrimp in the offshore area decrease, while the number of crabs might increase, which is likely to be the reason why BFS changes its feeding habits in Xinghua Bay.
Comparison of feeding habits of BFS in different wintering areas
Studies have shown that the feeding habits of BFS in different wintering areas are different. In Chinese Taiwan and Vietnam, fish and shrimp are the main food sources of BFS (Swennen and Yu 2005). In Hong Kong, fishes such as Mugiliformes, Cichliformes and Gobiiformes, are the main foods of BFS (Huang et al. 2021). Our results based on stable isotope analysis showed that Palaemonidae and Portunidae provide a greater contribution to the food composition of BFS in Xinghua Bay, which is quite different from other wintering areas. The reasons may include the different composition of food resources in both natural and artificial wetlands, and the latter is mainly caused by different management methods.
There are large areas of aquaculture ponds in the Chiku Wetland in Taiwan and Mai Po Wetland in Hong Kong, most of which are used to raise Mugilidae, Cyprinidae, and tilapia. By adjusting the appropriate water depth, these aquaculture ponds can provide foraging habitat for BFS. However, most of the ponds in Xinghua Bay are deep-water aquaculture ponds, which can only be used by BFS when drained for fishing close to the Chinese Spring Festival. At other times, the overwintering BFS population experiences difficulty using aquaculture ponds but prefers to forage in natural wetlands. In addition, because of the scarcity of fish resources in the intertidal zone of Xinghua Bay, BFS here prefers to feed on shrimp and crabs.
Relative utilization preference of BFS to natural wetlands and fishponds
The traditional, extensive fish ponds in Haifeng, Guangdong Province attract large numbers of waterbirds to spend the winter here every year, so this wetland is also an important wintering area for BFS (Hu et al. 2009). Zeng et al.’s (2019) research showed that maintaining the original ecological function of coastal wetlands can provide good habitat and rich food resources for BFS, while intensive aquaculture ponds have been separated from the tides, resulting in the degradation of wetland function. As a result, the ecological habits of BFS were limited by the loss of habitat (Zeng et al. 2019). According to Jin et al.’s (2009) field observations, the aquaculture ponds in Xinghua Bay are generally drained and cleaned in winter. At this time, the BFS stay on ponds to rest during the day and rarely look for food. Only at dusk does the BFS fly to nearby tidal wetlands to obtain food (Jin et al. 2009). Our research supports Jin et al.’s observation that BFS mainly rest on fishponds and fly to nearby tidal wetlands for food. Furthermore, we use direct dietary evidence to suggest that the foods of BFS in Xinghua Bay mainly come from natural wetlands rather than from fishponds.
The types of artificial wetlands that can be used by waterbirds include rice fields, salt ponds, aquaculture ponds, and reservoirs. Different types of artificial wetlands provide different ecological functions for waterbirds because of the different management modes. Because of the seasonal drainage of rice fields, such wetlands are often used by waterbirds such as egrets and herons as migration supply and wintering sites, while other waterbirds may use them as breeding habitats (Wood et al. 2013). Aquaculture and salt ponds can provide resting sites and alternative feeding grounds for shorebirds at high tide (Li et al. 2013). Because the management mode will deeply affect the carrying function of artificial wetlands on waterbird diversity, reasonable planning and management, especially controlling water depths (Ma et al. 2004), can effectively improve the supplementary function of artificial wetlands to natural wetlands. The Mai Po Nature Reserve in Hong Kong has adopted measures such as regulating the water levels of ponds, regularly desilting ponds, and pruning plants on embankments to create suitable habitats for large numbers of waterbirds (He et al. 2016). In Xinghua Bay, with the continuous deterioration in the quality of natural wetlands caused by the surrounding development, the existing aquaculture ponds should be retained and scientifically managed to alleviate the survival pressure for large numbers of wintering waterbirds, especially to protect rare species such as BFS.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.ACKNOWLEDGMENTS
We thank Xuan Wang, Leandro Bugoni, Sikai Wang, Jiling Zang, and Fengshan Liu for their assistance. We also thank Zixiang Gao, Xiangyu Pan, Lei Wu, and Mingxu Han for their sampling assistance. This research is funded by the National Natural Science Foundation of China (31700345) and the Bird Banding and Migration Research Project (first stage) (KH210257A) of the Mingxi County Forestry Bureau.
DATA AVAILABILITY
The data/code that support the findings of this study are available on request from the corresponding author, Ying Chen.
LITERATURE CITED
Boecklen, W. J., C. T. Yarnes, B. A. Cook, and A. C. James. 2011. On the use of stable isotopes in trophic ecology. Annual Review of Ecology, Evolution, and Systematics 42:411-440. https://doi.org/10.1146/annurev-ecolsys-102209-144726
Caut, S., E. Angulo, and F. Courchamp. 2009. Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. Journal of Applied Ecology 46:443-453. https://doi.org/10.1111/j.1365-2664.2009.01620.x
Chen, Y. S., and Y. Q. Xu. 1992. Study on the community ecology of crabs in the intertidal zone of Meihua port, Minjiang Estuary. [Translated from the Chinese by the authors.] Journal of Fujian Normal University (Natural Science) 8:4.
Choi, C., X. Gan, N. Hua, Y. Wang, and Z. Ma. 2014. The habitat use and home range analysis of Dunlin (Calidris alpina) in Chongming Dongtan, China and their conservation implications. Wetlands 34:255-266. https://doi.org/10.1007/s13157-013-0450-9
Gasperin, G., and M. A. Pizo. 2009. Frugivory and habitat use by thrushes (Turdus spp.) in a suburban area in south Brazil. Urban Ecosystems 12:425. https://doi.org/10.1007/s11252-009-0090-2
He, S. Y., T. Hu, H. L. Xu, and X. H. Shi. 2016. Experiences in conservation and management of Mai Po Nature Reserve of Hong Kong and their implications. [Translated from the Chinese by the authors.] Wetland Science & Management 12:26-29.
Hu, J. H., X. W. Zeng, Z. Y. Xie, and H. Y. Hu. 2009. Current status of wintering population of Black-faced Spoonbill in Haifeng Avian Natural Reserve, Guangdong. [Translated from the Chinese by the authors.] Chinese Journal of Zoology 44:54-57.
Huang, L. M., Y. Z. Zhang, X.S. Jin, and X. J. Shan. 2010. A comparative study of fish community in four main estuaries of China southeastern coastal areas and their adjacent waters. Journal of Ocean University of China 9:169-177. https://doi.org/10.1007/s11802-010-0169-7
Huang, P. Y., E. S. K. Poon, A. T. C. Wong, I. W. Y. So, Y. H. Sung, and S. Y. W. Sin. 2021. DNA metabarcoding reveals the dietary composition in the endangered Black-faced Spoonbill. Scientific Reports 11:18773. https://doi.org/10.1038/s41598-021-97337-w
Inger, R., and S. Bearhop. 2008. Applications of stable isotope analyses to avian ecology. Ibis 150:447-461. https://doi.org/10.1111/j.1474-919X.2008.00839.x
Jeong, M. S., C. Y. Choi, W. S. Lee, and K. S. Lee. 2021. Age-dependent shifts and spatial variation in the diet of endangered Black-faced Spoonbill (Platalea minor) chicks. PLoS ONE 16:e0253469. https://doi.org/10.1371/journal.pone.0253469
Jin, J. F., B. F. Liu, X. Yu, and C. H. Lu. 2009. Wintering and migration of Black-faced Spoonbill in Xinghua Bay, Fujian Province. [Translated from the Chinese by the authors.] Chinese Journal of Zoology 44:47-53.
Jin, J. F., B. F. Liu, X. Yu, and C. H. Lu. 2010. Fish and shrimps in feeding habitat of Platalea minor in Xinghua Bay, Fujian, China. [Translated from the Chinese by the authors.] Chinese Journal of Zoology 45(2):69-74.
Li, D., S. Chen, H. Lloyd, S. Zhu, K. Shan, and Z. Zhang. 2013. The importance of artificial habitats to migratory waterbirds within a natural/artificial wetland mosaic, Yellow River Delta, China. Bird Conservation International 23:184-198. https://doi.org/10.1017/S0959270913000099
Li, J., J. R. Luo, X. H. Li, X. C. Tan, C. Wang, and S. C. Guo. 2007. Investigation of fish resources and analysis of resource decline along Lianjiang River. [Translated from the Chinese by the authors.] Freshwater Fisheries 37(3):49-53.
Li, J. R., L. X. Li, and L. Yang. 2016. Pretreatment methods for stable isotopic analysis of avian tissues. [Translated from the Chinese by the authors.] Chinese Journal of Zoology 51(3):477-486.
Li, R. G., J. X. Jiang, E. X. Chai, H. Z. Xu, Q. Q. Wu, and S. D. Lin. 1999. Study on macrobenthos ecology in Xinhua Bay. [Translated from the Chinese by the authors.] Acta Oceanologica Sinica 21(5):101-109.
Lilliendahl, K., and J. Solmundsson. 2006. Feeding ecology of sympatric European Shags Phalacrocorax aristotelis and great cormorants P. carbo in Iceland. Marine Biology 149:979-990. https://doi.org/10.1007/s00227-006-0259-7
Liu, B. F. 2006. Distribution of Black-faced Spoonbill and its habitats in Fujian Province. [Translated from the Chinese by the authors.] Chinese Journal of Zoology 41:48-52.
Lou, Z. K., Y. Li, W. Liu, and X. Y. Li. 2019. Differences in biodiversity of diural and nocturnal waterbirds between natural and artificial wetlands: a case study from Caohai Nature Reserve and Yangwanqiao Reservoir, Guizhou Province. [Translated from the Chinese by the authors.] Journal of East China Normal University (Natural Science) 2019(3):120-130.
Luo, X. X. 2018. Preliminary investigation of fish resources in the lower reaches of Min River. [Translated from the Chinese by the authors.] Journal of Subtropical Resources and Environment 13:5.
Luo, Z. K., W. H. Zhang, Y. Hou, Y. L. Xing, L. Wen, and Z. J. Li. 2013. Seasonal dynamics and habitat selection of Ruddy Shelduck (Tadorna ferruginea) (Anseriformes: Anatidae) in alpine wetland ecosystem of southwest China. Acta Zoologica Bulgarica 65:469-478.
Ma, Z., B. Li, B. Zhao, K. Jing, S. Tang, and J. Chen. 2004. Are artificial wetlands good alternatives to natural wetlands for waterbirds? A case study on Chongming Island, China. Biodiversity & Conservation 13:333-350. https://doi.org/10.1023/B:BIOC.0000006502.96131.59
Masero, J. A. 2003. Assessing alternative anthropogenic habitats for conserving waterbirds: salinas as buffer areas against the impact of natural habitat loss for shorebirds. Biodiversity & Conservation 12:1157-1173. https://doi.org/10.1023/A:1023021320448
Nielsen, J. M., E. L. Clare, B. Hayden, M. T. Brett, and P. Kratina. 2018. Diet tracing in ecology: method comparison and selection. Methods in Ecology and Evolution 9:278-291. https://doi.org/10.1111/2041-210X.12869
Parnell, A. C., R. Inger, S. Bearhop, and A. L. Jackson. 2010. Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5:e9672. https://doi.org/10.1371/journal.pone.0009672
Parnell, A. C., D. L. Phillips, S. Bearhop, B. X. Semmens, E. J. Ward, J. W. Moore, A. L. Jackson, J. Grey, D. J. Kelly, and R. Inger. 2013. Bayesian stable isotope mixing models. Environmetrics 24:387-399. https://doi.org/10.1002/env.2221
Pickett, E. J., M. Chan, W. Cheng, J. Allcock, S. Chan, J. Hu, K. Lee, B. Smith, S. Xing, Y.-T. Yu, and T. C. Bonebrake. 2018. Cryptic and cumulative impacts on the wintering habitat of the endangered Black-faced Spoonbill (Platalea minor) risk its long-term viability. Environmental Conservation 45:147-154. https://doi.org/10.1017/S0376892917000340
Rajpar, M. N., and M. Zakaria. 2013. Assessing an artificial wetland in Putrajaya, Malaysia, as an alternate habitat for waterbirds. Waterbirds 36:482-493. https://doi.org/10.1675/063.036.0405
Ruan, Y. Q., B. F. Liu, X. Yu, S. L. Song, D. T. Zheng, Y. M. Chen, X. Y. Wang, and M. C. Lin. 2006. Ecological study of overwintering Black-faced Spoonbill and conservation strategy. [Translated from the Chinese by the authors.] Chinese Wildlife 27:4.
Swennen, C. K., and Y. T. Yu. 2005. Food and feeding behavior of the Black-faced Spoonbill. Waterbirds 28:19-27. https://doi.org/10.1675/1524-4695(2005)028[0019:FAFBOT]2.0.CO;2
Takano, S., F. Takeshita, and Y. Henmi. 2014. Spatiotemporal utilization of feeding sites by the Black-faced Spoonbill Platalea minor in the Yatsushiro Sea, Japan. Ornithological Science 13:59-66. https://doi.org/10.2326/osj.13.59
Ueng, Y. T., J. J. Perng, J. P. Wang, J. H. Weng, and P. C. Hou. 2006. Diet of the Black-faced Spoonbill wintering at Chiku Wetland in southwestern Taiwan. Waterbirds 29:185-190. https://doi.org/10.1675/1524-4695(2006)29[185:DOTBSW]2.0.CO;2
Wang, R., and Y. Zhang. 2012. Survey on the wintering waterbirds of coastal Fujian. [Translated from the Chinese by the authors.] Chinese Journal of Wildlife 33:271-274.
Wang, X., H. X. Jiang, and Y. N. Zhang. 2015. Application of stable isotope analyses to avian diets and trophic structure. [Translated from the Chinese by the authors.] Acta Ecologica Sinica 35:14. https://doi.org/10.5846/stxb201402120243
Wang, X., J. Hongxing, Z. Yanan, C. Lixia, S. Changzhan, and L. Yuxiang. 2017. Diet composition of Saunders’s Gull (Larus saundersi) determined using stable isotopic analysis at the Shuangtaihekou National Nature Reserve, China. [Translated from the Chinese by the authors.] Acta Ecologica Sinica 37:1796-1804. https://doi.org/10.5846/stxb201511092268
Wood, C., H. Tomida, K. Jin-Han, K. S. Lee, H. J. Cho, S. Nishida, J. Ibrahim, W. H. Hur, H. J. Kim, S. H. Kim, H. Koike, G. Fujita, H. Higuchi, and T. Yahara. 2013. New perspectives on habitat selection by the Black-faced Spoonbill Platalea minor based upon satellite telemetry. Bird Conservation International 23:495-501. https://doi.org/10.1017/S0959270913000105
Wu, J. W., Z. Li, H. S. Lin, K. Liu, and Y. Q. Huang. 2019. Community structure and temporal and spatial pattern of fouling organisms in Xinghua Bay. [Translated from the Chinese by the authors.] Journal of Applied Oceanography 38:7.
Xia, S., X. Yu, S. Millington, Y. Liu, Y. Jia, L. Wang, X. Hou, and L. Jiang. 2017. Identifying priority sites and gaps for the conservation of migratory waterbirds in China’s coastal wetlands. Biological Conservation 210:72-82. https://doi.org/10.1016/j.biocon.2016.07.025
Xu, Z., and Y. Sun. 2013. Comparison of shrimp density between the Minjiang Estuary and Xinghua Bay during spring and summer. [Translated from the Chinese by the authors.] Acta Ecologica Sinica 33:9. https://doi.org/10.5846/stxb201207261060
Yu, Y. T., C. H. Li, I. W. L. Tse, and H. H. N. Fong. 2021. International Black‑faced Spoonbill census 2021. Black‑faced Spoonbill Research Group, the Hong Kong Bird Watching Society, Hong Kong, China.
Yu, Y. T. and C. K. Swennen. 2004. Habitat use of the Black-faced Spoonbill. Waterbirds 27:129-134. https://doi.org/10.1675/1524-4695(2004)027[0129:HUOTBS]2.0.CO;2
Zeng, X. W., Y. Z. Lin, H. J. Luo, L. F. Yao, and X. Q. Liu. 2019. Correlation between aquaculture and population changes of Wintering Black faced Spoonbill in Haifeng County. [Translated from the Chinese by the authors.] Journal of Green Science and Technology 2019(12):41-43.
Zhang, H., J. C. Wu, and P. He. 2019. Investigation on the distribution of waterfowl in Xinghua Bay, Fuqing. [Translated from the Chinese by the authors.] Forestry Prospect and Design 39:77-79.
Zhong, S. L., C. Y. Xu, M. F. Yang, S. C. Jiang, S. H. Zheng, and H. D. Zheng. 2011. Research status and development trend of marine fishery ecological environment in Fujian. [Translated from the Chinese by the authors.] Journal of Fujian Fisheries 33:52060.
Fig. 1
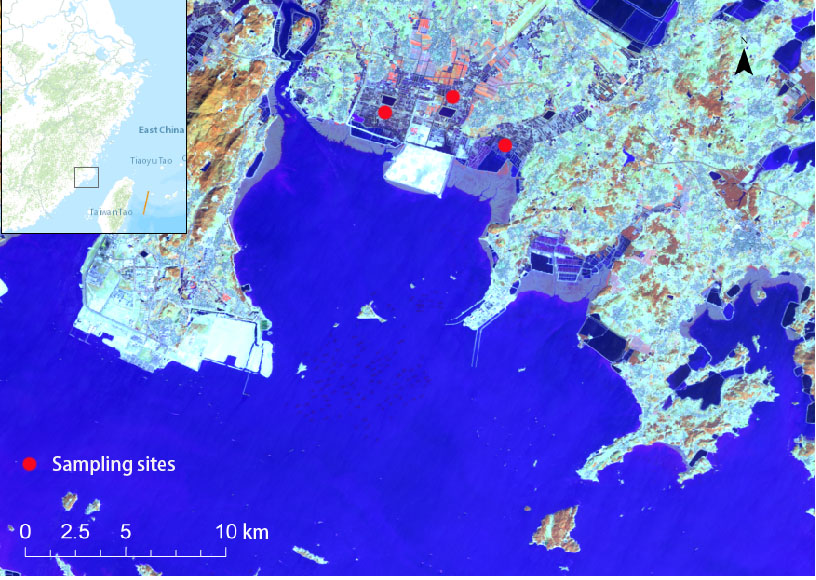
Fig. 1. Fecal and potential food sampling sites in Xinghua Bay. Figure created in ArcGIS Version 10.4.1.

Fig. 2
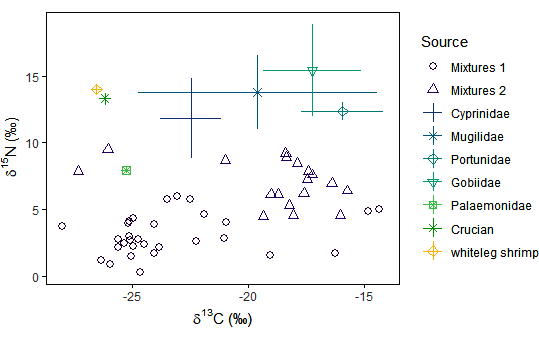
Fig. 2. The distributions of stable isotope values in Black-faced Spoonbill (Platalea minor; BFS) feces and their potential foods. Mixtures 1 represents the stable isotope values of BFS feces in early winter, and Mixtures 2 represents the values in late winter.

Fig. 3
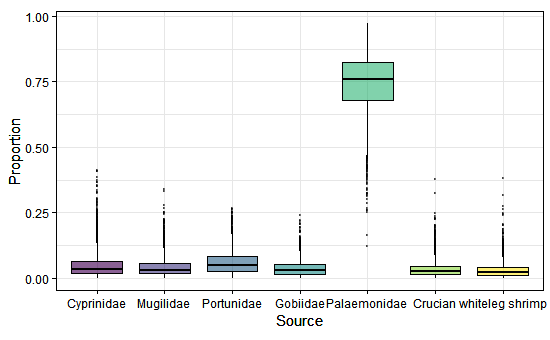
Fig. 3. Represents the contribution rates of seven potential foods in early winter.

Fig. 4
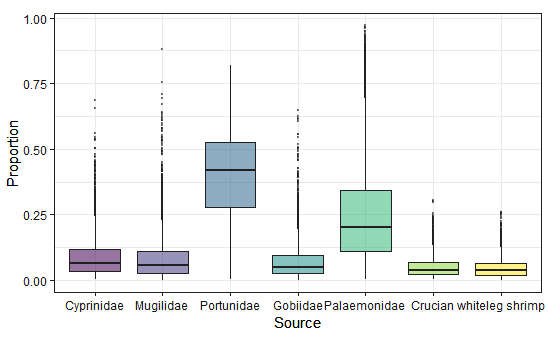
Fig. 4. Represents the contribution rates of seven potential foods in late winter.

Table 1
Table 1. Mean and standard deviation values of foods and feces after correction. BFS = Black-faced Spoonbill (Platalea minor).
| Source | Samples | Number | δ13C/‰ | δ15N/‰ |
| Natural wetland | Cyprinidae | 3 | -22.48±1.32 | 11.80±3.01 |
| Mugilidae | 7 | -19.62±5.15 | 13.76±2.75 | |
| Portunidae | 4 | -15.95±1.76 | 12.34±0.67 | |
| Gobiidae | 5 | -17.26±2.21 | 15.41±3.45 | |
| Palaemonidae | 3 | -25.28±0.22 | 7.92±0.20 | |
| Fishponds | Crucian | 2 | -26.20±0.03 | 13.30±0.18 |
| Whiteleg shrimp | 2 | -26.55±0.01 | 13.98±0.01 | |
| BFS | Feces (early winter) | 31 | -23.28±3.30 | 3.08±1.50 |
| Feces (late winter) | 26 | -18.92±3.10 | 6.99±1.63 | |
Table 2
Table 2. Contribution rates of the potential foods of Black-faced Spoonbill (Platalea minor) in Xinghua Bay.
| Species | Contribution rates (early winter) |
Contribution rates (late winter) |
||
| Mean (%) ± SD | 95% CI | Mean (%) ± SD | 95% CI | |
| Cyprinidae | 5.1±0.05 | 0.5-18.6 | 8.8±0.08 | 0.8-31.5 |
| Mugilidae | 4.2±0.04 | 0.4-13.9 | 8.5±0.09 | 0.7-34.3 |
| Portunidae | 6.0±0.05 | 0.6-16.9 | 39.3±0.18 | 2.1-67.4 |
| Gobiidae | 3.7±0.03 | 0.4-11.6 | 7.3±0.07 | 0.7-27.5 |
| Palaemonidae | 74.4±0.11 | 49.1-91.4 | 26.1±0.22 | 2.1-85.4 |
| Crucian | 3.0±0.03 | 0.4-11.8 | 5.1±0.04 | 0.6-16.0 |
| Whiteleg shrimp | 3.0±0.03 | 0.4-10.6 | 4.8±0.04 | 0.6-14.8 |









